Published online Jun 21, 2005. doi: 10.3748/wjg.v11.i23.3595
Revised: June 8, 2004
Accepted: June 29, 2004
Published online: June 21, 2005
AIM: To investigate the differences in biological features of gastric dysplasia (Dys), indefinite dysplasia (IDys) and reactive hyperplasia (RH) by studying the biomarker alterations in cell proliferation, cell differentiation, cell cycle control and the expression of house-keeping genes, and further to search for markers which could be used in guiding the pathological diagnosis of three lesions.
METHODS: Expressions of MUC5AC, MUC6, adenomatous polyposis coli (APC), p53, Ki-67, proliferation cell nuclear antigen (PCNA) and EGFR were studied by immunohistochemistry with a standard Envision technique in formalin-fixed and paraffin-embedded specimens from 43 RH, 35 IDys, 35 Dys and 36 intestinal type gastric carcinomas (IGC). In addition, Bayes discriminant analysis was used to investigate the value of markers studied in differential diagnosis of RH, IDys, Dys and IGC.
RESULTS: The MUC5AC and MUC6 antigen expressions in RH, IDys, Dys and IGC decreased gradually (MUC5AC: 86.04%, 77.14%, 28.57%, 6.67%; MUC6: 65.15%, 54.29%, 20.00%, 25.00%, respectively). The expressions of the two markers had no significant difference between RH and IDys, but were all significantly higher than those of the other two lesions (MUC5AC: χ2 = 27.607, 38.027 and 17.33, 26.092; MUC6: χ2 = 16.54, 12.665 and 9.282, 6.737, P<0.01). There was no significant difference between RH and IDys, Dys and IGC in MUC6 expression. The APC gene expression in the four lesions had a similar decreasing tendency (RH 69.76%, IDys 68.57%, Dys 39.39%, IGC 22.86%), and it was significantly higher in the first two lesions than in the last two (χ2 = 7.011, 16.995 and 14.737, 19.817, P<0.05). The p53 expression in RH, IDys, Dys and IGC was 6.98%, 20%, 57.14% and 50%, respectively. There was no significant difference between RH and IDys or Dys and IGC, but the p53 expression in RH and IDys was significantly lower than that in Dys and IGC (χ2 = 7.011, 16.995 and 14.737, 19.817, P<0.01). The Ki-67 label index was significantly different among four lesions (RH: 0.298±8.92%, IDys: 0.358±9.25%, Dys: 0.498±9.03%, IGC: 0.620±10.8%, P<0.001). Positive immunostaining of PCNA was though observed in all specimens, significant differences were detected among four lesions (F = 95.318, P<0.01). In addition, we used Bayes discriminant analysis to investigate molecular pathological classification of the lesions, and obtained the best result with the combination of MUC5AC, Ki-67 and PCNA. The overall rate of correct classification was 67.4% (RH), 68.6% (IDys), 70.6% (Dys) and 84.8% (IGC), respectively.
CONCLUSION: Dys has neoplastic biological characteristics, while RH and IDys display hyperplastic characteristics. MUC5AC and proliferation-related biomarkers (Ki-67, PCNA) are more specific in distinguishing Dys from RH and IDys.
- Citation: Dong B, Xie YQ, Chen K, Wang T, Tang W, You WC, Li JY. Differences in biological features of gastric dysplasia, indefinite dysplasia, reactive hyperplasia and discriminant analysis of these lesions. World J Gastroenterol 2005; 11(23): 3595-3600
- URL: https://www.wjgnet.com/1007-9327/full/v11/i23/3595.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i23.3595
Gastric indefinite dysplasia (IDys) and reactive hyperplasia (RH) are common hyperplastic lesions of stomach mucosa in Padova international classification (1998)[1]. Gastric dysplasia (Dys) is a precancerous lesion, a non-invasive neoplasia or intraepithelial neoplasma (IEN), which is divided into low grade (LGD) and high grade (HGD). In Padova classification, morphological description is adopted for classification and diagnosis of these lesions. However, the description is simple and often poses difficulties for pathologists to differentiate RH from IDys or IDys from Dys, and the differential diagnosis of IDys and LGD is especially difficult to make. Up to now, only a few systemic investigations have been made on the biological characteristics of these lesions. Therefore, it is necessary to precisely and clearly discriminate RH, IDys and Dys so as to diagnose them accurately and to treat them efficiently. The main purpose of this study was to investigate the biological differences among Dys, IDys and RH, and to further combine them with their morphological descriptions, to search for clear and external markers, which could be used in the pathological diagnosis of the three lesions.
From the pathologic files at the Pathology Department of Beijing Cancer Hospital, 36 intestinal type gastric carcinomas (IGCs) surgically resected at Beijing Cancer Hospital between 1998 and 2002 were collected. Other lesions including RH, IDYs and gastric Dys came mostly from mucosae near the carcinomas in 60 patients. Twenty-two gastric Dys lesions were collected from the People’s Hospital of Linqu County in Shandong Province by endoscopic biopsy between 1996 and 2001. A total of 149 specimens from 88 patients were used in our study. Among these specimens, 43 had RH (27 males and 16 females, age range 27-84 years), 35 had IDys (21 males and 14 females, age range 38-81 years), 35 had Dys (24 males and 11 females, age range 40-70 years) and 36 had IGC (25 males and 11 females, age range 36-75 years). All specimens were checked by at least two pathologists. To rule out any effect of sex and age on the biological behaviors of the lesions, we did statistical analysis and found that sex and age had no effect on the biological features.
MUC5AC (NeoMarkers, MS-145-P0 45M1), MUC6 (NeoMarkers, MS-1153-S0 MCN6.01/CLH5) and p53 (MAB-0142 DO-7) were purchased from Maxim Biotech, Inc. APC (EMM43) was obtained from Novacastra Laboratories Ltd. EGFR (H11 M3563) was purchased from Dako (1:60 dilution) and Ki-67 (MAB-0129 MIB-1) from Maxim Biotech Inc. Proliferation cell nuclear antigen (PCNA) (ZM-0213 PC10) (1:100 dilution) was from Zhongshan Biotechnology Co., Ltd. Secondary antibody of IgG anti-Fab-HRP and PV-6000 PicTureTM Kits were from Zymed Laboratory Co., Ltd.
Five-micrometer-thick tissue sections were dewaxed with xylene and rehydrated with graded alcohol, then briefly immersed in water. Endogenous peroxidase activity was blocked by incubating the sections with 3% hydrogen peroxide for 10 min. For all biomarkers, except for MUC5AC, heat-mediated antigen retrieval was performed by heating the sections (immersed in 0.01 mol/L citrate buffer, pH 6.0) in a microwave oven (450 W) for 10 min. The slides were then washed with phosphate-buffered saline (PBS) before incubation with respective primary antibody overnight at 4 °C. After being washed with PBS, the slides were incubated for 1 h with the secondary antibody, and then further washed for 3 min×5 min with PBS. Peroxidase reaction was developed in PBS using hydrogen peroxide as a substrate and DAB as a chromogen. Sections were counterstained with hematoxylin, dehydrated, and evaluated under a light microscope. Negative control sections were processed immunohistochemically after the primary antibody was replaced with PBS.
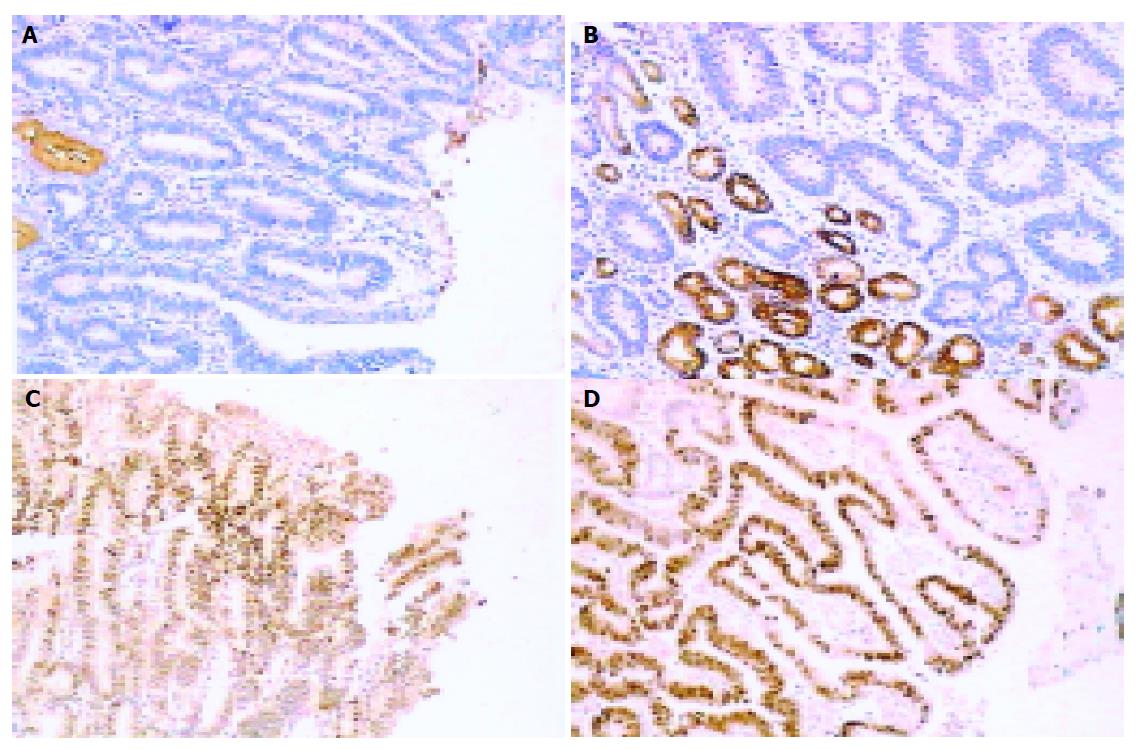
To study the biological features, we used MUC5AC and MUC6 for cell differentiation, p53 for cell cycle control, Ki-67, PCNA and EGFR for proliferation, APC for house-keeping gene abnormality. MUC5AC was highly expressed in foveolar epithelium and mucous neck cells of antrum and body regions. Expression of MUC6 was detected in the glands of the antrum and mucopeptic cells in the neck zone of the normal gastric body region[2]. Only nuclear immunostaining was considered as positive for p53 protein accumulation[3-6]. EGFR and APC positive staining were located on cytoplasms and cell membranes. Sections were classified according to the immunostaining into negative group (less than 10% of positive cells) and positive (positive in more than 10% of positive cells)[4,7]. With regard to the Ki-67 labeling index (LI), only nuclear staining was evaluated as positive. We randomly selected four high-power (400×) microscopic fields and counted 500 cells in each section, and then determined the average Ki-67 LI[8-11].
Only cells showing nuclear staining were considered to be positive for PCNA and graded as negative (-) (no positive cells); weak positive (+), positive cells accounted for 1-25%; moderately positive (++), the positive cells accounted for 26-50%; strong positive (+++), the positive cells accounted for 51-75%; very strong positive (++++), the positive cells accounted for greater than >75%[12].
The χ2 test and one-way ANOVA test were used to compare the frequencies by SPSS 10.0 for Windows. P<0.05 was considered statistically significant. In addition, we used Bayes discriminant analysis to investigate the molecular pathological classification of RH, IDys, Dys and IGC. Bayes discriminant analysis was used to identify the combined effect of variables important for discrimination of different lesions. Misclassification was evaluated by the leave-one-out cross-validation technique. Analyses were performed using SPSS 10.0.
Using a set of seven variables, discriminant analysis correctly classified lesions into one of the following three groups: left-sided RH, right-sided, Dys.
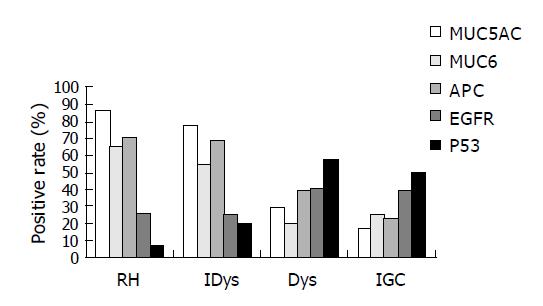
MUC5AC positive expression in each kind of lesions was 86.4% (37/43) for RH, 77.14% (27/35) for IDys, 28.57% (10/35) for Dys and 16.67% (6/36) for IGC. The percentage of RH and IDys with positive staining for MUC5AC was significantly higher than that in other lesions (χ2 = 27.607, 38.027 and 17.33, 26.092, P<0.01) (Figures 1 and 2).
MUC6 positive expression in each kind of lesion was 65.15% (28/43) for RH, 54.29% (19/35) for IDys, 20.00% (7/35) for Dys and 25.00% (9/36) for IGC. MCU6 positive expression had no difference in RH and IDys, but was significantly higher than that in the other two lesions (χ2 = 16.54, 12.665 and 9.282, 6.737, P<0.01), and no significant difference was observed between Dys and IGC (Figures 1 and 2).
APC positive expression in each kind of lesions was 69.76% (30/43) for RH, 68.57% (24/35) for IDys, 39.39% (13/33) for Dys and 22.86% (8/35) for IGC. APC positive expression had no difference in RH and IDys, but was significantly higher than that in the other two lesions (χ2 = 7.011, 16.995 and 14.737, 19.817, P<0.05), and no significant difference were observed between Dys and IGC (Figure 1).
EGFR positive expression in each kind of lesions was 26.19% (11/42) for RH, 25.00% (8/35) for IDys, 40.00% (14/35) for Dys and 38.89% (14/36) for IGC. EGFR positive expression had no difference in each group (Figure 1).
p53 positive expression in each kind of lesions was 6.98% (3/43) for RH, 20.00% (7/35) for IDys, 57.14% (20/35) for Dys and 50.00% (18/36) for IGC. For p53 expression, there was no relationship between RH and IDys. The p53 expression observed between Dys and IGC had no significant difference either, but both of these lesions had a significantly higher p53 expression than that detected in the nuclei of RH and IDys (χ2 = 7.011, 16.995 and 14.737, 19.817, P<0.05) (Figures 1 and 2).
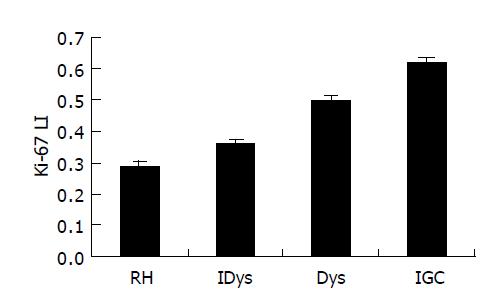
In normal gastric mucosa, Ki-67 immunoreactivity was mainly found in foveolar hyperproliferative zone. The mean Ki-67 index (mean±SE) was 0.298±8.92% for RH, 0.358±9.25% for IDys, 0.498±9.03% for Dys, and 0.620±10.8% for IGC. The Ki-67 index for IGC was significantly higher than that of the other three lesions (P<0.001). There was also a significant difference in RH, IDys and Dys (eP<0.01) (Figure 3). In addition, there was no locus restriction in Dys and IGC (Figure 2).
All lesions showed a different extent of positive immunostaining of PCNA protein (Table 1). In normal gastric mucosa, it was mainly in foveolar hyperproliferative zone. Positive immunostaining was observed in the cell nuclei of tumor and epithelial tissues. There were significant differences among RH, IDys, Dys, and IGC (P<0.01). Additionally, PCNA expression had no locus restriction in Dys and IGC.
| PCNA expression | RH | IDys | Dys | IGC |
| - | 0 | 0 | 0 | 0 |
| + | 25 | 9 | 0 | 0 |
| ++ | 17 | 24 | 9 | 1 |
| +++ | 1 | 2 | 15 | 11 |
| ++++ | 0 | 0 | 11 | 24 |
| Total | 43 | 35 | 35 | 36 |
PCNA expression was graded as negative (-) (no positive cells); weak positive (+), positive cells accounted for 1-25%; moderately positive (++), positive cells accounted for 26-50%; strong positive (+++), positive cells accounted for 51-75%; very strong positive (++++), positive cells accounted for greater than 75%.
We made discriminant analysis for seven parameters to find out which variables would best classify the lesions and found that the best result was obtained with the combination of MUC5AC, Ki-67 and PCNA. The overall rate of correct classification was 67.4% (RH), 68.6% (IDys), 70.6% (Dys) and 84.8% (IGC) respectively (Table 2).
| Membership | Predicted group | Total | ||||
| PATH | RH | IDys | Dys | IGC | ||
| Original Count | RH | 29 (67.4) | 13 (30.2) | 1 (2.3) | 0 (0.0) | 43 |
| IDys | 9 (25.7) | 24 (68.6) | 1 (5.7) | 0 (0.0) | 35 | |
| Dys | 0 (0.0) | 3 (8.8) | 24 (70.6) | 7 (20.6) | 34 | |
| IGC | 0 (0.0) | 1 (3.0) | 4 (12.1) | 28 (84.8) | 33 | |
| Ungrouped cases | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 | |
| Cross-validated Count | RH | 28 (69.8) | 12 (32.6) | 1 (2.3) | 0 (0.0) | 43 (100.0) |
| IDys | 14 (40.0) | 20 (54.3) | 1 (5.7) | 0 (0.0) | 35 (100.0) | |
| Dys | 0 (0.0) | 4 (11.8) | 24 (67.6) | 7 (20.6) | 35 (100.0) | |
| IGC | 0 (0.0) | 3 (3.0) | 19 (15.2) | 47 (81.8) | 69 (100.0) | |
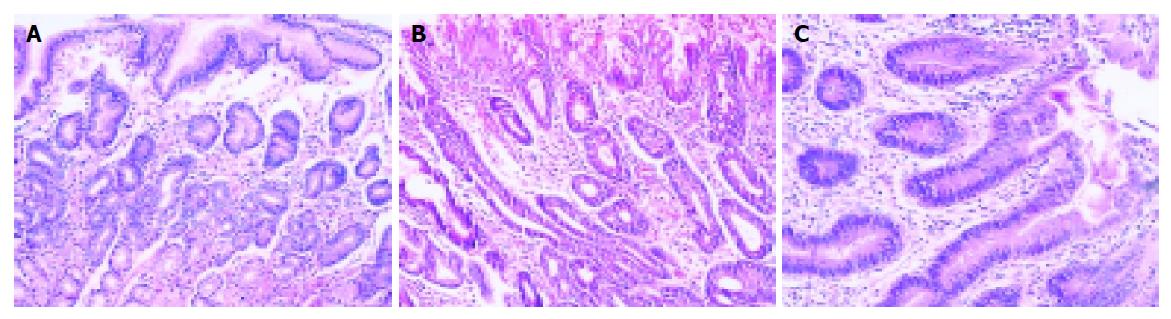
Compared with the other three lesions, RH preserved most characteristics of normal gastric mucosa (Figure 4A), and its cell differentiation, house-keeping gene abnormality, cell cycle control and proliferation had minimal abnormalities. This was supported by the fact that the expression rates of MUC5AC[13], MUC6[14,15] (two biomarkers for cell differentiation) and APC (a house-keeping gene) were the highest, its p53 (a biomarker for cell cycle control) expression rate was the lowest, and its Ki-67 LI and PCNA expressions (two biomarkers for cell proliferation) were the lowest. We also saw that RH was significantly different from Dys and IGC in expression of all biomarkers except EGFR, suggesting that RH might be of an infectious nature. Therefore, taking its morphological characteristics into consideration, it is an inflammatory lesion.
This category was reserved in cases in which a pathologist was unable to decide for certain if the lesion could represent neoplastic or non-neoplastic cells. This situation might arise because the biopsy materials were inadequate or because the architectural distortion and nuclear atypia were present at the point of creating doubts about the dysplastic nature of proliferating cells. In such cases the doubts might be solved with more adequate new biopsies or after possible sources of cellular hyperproliferation or atypia were removed, such as H pylori or nonsteroidal anti-inflammatory drugs[16,17]. In Padova classification, it emphasizes that the “gradient of maturation” must been seen in IDys, which means that the structural and cellular alterations tend to decrease from the bottom to the most superficial mucosal layers (Figure 4B).
IDys has two subtypes, namely, foveolar hyperproliferation and hyperproliferative intestinal metaplasia. In our study, the p53 and PCNA positive expression rates were just a little bit higher than those of RH, but significantly lower than those of Dys and IGC (P<0.05). Its Ki-67 LI level was lower than that of Dys and IGC (P<0.01), though still higher than that of RH (P<0.01). The MUC5AC, MUC6 and APC positive expression rates were a little bit lower than those of RH, but significantly higher than those of Dys and IGC (P<0.05). Results showed that in IDys, although the ability of cell proliferation was increased, it was still relatively low. IDys still kept many characteristics which were similar to those of normal gastric mucosa, such as cell differentiation and proliferation, cell cycle control and APC gene expression. It was significantly different from Dys and IGC in these areas, but was similar to RH. Therefore, IDys essentially belongs to benign hyperplastic lesions.
Commonly seen in clinics, this lesion was often difficult to diagnose and could lead to diverging opinions. Consequently, clinicians had difficulties in treating it. In Padova classification, no further illumination is provided to this lesion’s essence either. Based on our study, we conclude that IDys is a benign hyperplastic lesion. Therefore, when we see the “gradient of maturation” in morphology, we should consider the lesion as a benign hyperplastic one.
Dysplastic cells extended to the surface epithelium, a feature absent in non-neoplastic lesions. Because it was frequently absent in cell maturation, we could not see the “gradient of maturation” in Dys. Dys was divided into two subcategories: LGD and HGD (Figure 4C).
Our study revealed that there were obvious abnormalities in cell differentiation and proliferation, and APC gene expression in Dys. These abnormalities were similar to those of IGC, but distinct from those in RH and IDys. In our study, its MUC5AC[18-20], MUC6[21-23] and APC[24] positive expression rates were significantly lower than those of RH and IDys, but were not significantly different from those of IGC. p53 positive expression rate of Dys was significantly higher than those of RH and IGC, but not significantly different from that of IGC. Its Ki-67 LI and PCNA positive expression rates were significantly higher than those of RH and IDys, but significantly lower than those of IGC. Dys had the highest EGFR positive expression rate in the four lesions, but had no significant difference in other lesions (P>0.05). Although Dys was similar to IGC in its biological characteristics, the proliferation activity of IGC was higher than that of Dys, and was invasive to deeper layers of gastric mucosa. Thus, it is reasonable to categorize Dys as a non-invasive neoplasia or an IEN.
In our study, IGC was used as a positive control for the other three lesions. Its MUC5AC, MUC6 and APC[24] positive expression rates were significantly lower than those of RH and IDys and its p53 positive expression rate was significantly higher than that of RH and IDys, but the activity of four markers in IGC had no significant differences when compared with Dys. The Ki-67 LI and PCNA positive expression rates in IGC were significantly higher than those in the other three lesions. All these indicate that cell differentiation in IGC was the poorest, the abnormality in cell cycle control was the greatest and the proliferative activity was the highest in the four lesions. Its EGFR positive expression rate was higher than that of RH and IDys, lower than that of Dys. But there were no significant differences in each group (P>0.05). EGFR positive expression was low in all the four lesions.
It is well known that the use of discriminant analysis on a single data set could establish a model. That is, “over-fit” and use of the same discriminant functions on a new set of data usually produced much lower correct classification percentage. Thus, we assessed the misclassifications by leave-one-out cross validation. Bayes stepwise discriminant analysis could overcome the shortcomings of the general discriminant function by selecting variables, which have a significant influence on the functions. Thus, it could classify one case effectively. In clinical application, as data may vary greatly, that the functions are considered to be effective when the correct rate of discriminant functions is higher than 70%.
RH, IDys and Dys are different in their biological nature, and it is important to combine their biological nature with morphologic features in their diagnosis and differential diagnosis.
In the seven indexes used in our study, MUC5AC, PCNA and Ki-67 could best differentiate the four lesions. Besides, these markers were helpful in understanding the biological nature of these lesions. The correct rates of classification were 67.4% (RH), 68.6% (IDys), 70.6% (Dys), and 84.8% (IGC), respectively. The misclassifications mainly occurred in RH and IDys, or in Dys and IGC. The correct rate of the discriminant functions for Dys and IGC were higher than 70%. Therefore, if we encounter a gastric lesion which is difficult to diagnose, MUC5AC, Ki-67 and PCNA immunohistochemical assay and the discriminant model might be helpful for distinguishing Dys from RH and IDys.
Co-correspondents: Wei-Cheng You
| 1. | Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 240] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, Mandel U, Clausen H, Sobrinho-Simões M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003-1007. [PubMed] [Cited in This Article: ] |
| 3. | Ohbu M, Kobayashi N, Okayasu I. Expression of cell cycle regulatory proteins in the multistep process of oesophageal carcinogenesis: stepwise over-expression of cyclin E and p53, reduction of p21(WAF1/CIP1) and dysregulation of cyclin D1 and p27(KIP1). Histopathology. 2001;39:589-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Shirakawa Y, Naomoto Y, Kimura M, Kawashima R, Yamatsuji T, Tamaki T, Hamada M, Haisa M, Tanaka N. Topological analysis of p21WAF1/CIP1 expression in esophageal squamous dysplasia. Clin Cancer Res. 2000;6:541-550. [PubMed] [Cited in This Article: ] |
| 5. | Krecicki T, Jeleń M, Zalesska-Krecicka M, Rak J, Szkudlarek T, Jeleń-Krzeszewska J. Epidermal growth factor receptor (EGFR), proliferating cell nuclear antigen (PCNA) and Ki-67 antigen in laryngeal epithelial lesions. Oral Oncol. 1999;35:180-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Elpek GO, Gelen T, Aksoy NH, Karpuzoglu T, Keles N. Microvessel count, proliferating cell nuclear antigen and Ki-67 indices in gastric adenocarcinoma. Pathol Oncol Res. 2000;6:59-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Kase S, Osaki M, Honjo S, Adachi H, Ito H. Tubular adenoma and intramucosal intestinal-type adenocarcinoma of the stomach: what are the pathobiological differences? Gastric Cancer. 2003;6:71-79. [PubMed] [Cited in This Article: ] |
| 8. | Tao YS, Zong YS. The significance of over expression of P53 protein and proliferation cell nuclear antigen in esophageal epitheliosis and carcinogenesis. Carcinogenesis Teratogenesis Mutagenesis. 2002;14:87-90. [Cited in This Article: ] |
| 9. | De Bolós C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 213] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Morgenstern S, Koren R, Fraser G, Okon E, Niv Y. Gastric corpus mucin expression after partial gastrectomy, in relation to colonization with Helicobacter pylori. J Clin Gastroenterol. 2001;32:218-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 84] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Correa P, Ruiz B, Shi TY, Janney A, Sobhan M, Torrado J, Hunter F. Helicobacter pylori and nucleolar organizer regions in the gastric antral mucosa. Am J Clin Pathol. 1994;101:656-660. [PubMed] [Cited in This Article: ] |
| 13. | El-Zimaity HM, Genta RM, Graham DY. Histological features do not define NSAID-induced gastritis. Hum Pathol. 1996;27:1348-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Reis CA, David L, Nielsen PA, Clausen H, Mirgorodskaya K, Roepstorff P, Sobrinho-Simões M. Immunohistochemical study of MUC5AC expression in human gastric carcinomas using a novel monoclonal antibody. Int J Cancer. 1997;74:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 15. | Baldus SE, Mönig SP, Arkenau V, Hanisch FG, Schneider PM, Thiele J, Hölscher AH, Dienes HP. Correlation of MUC5AC immunoreactivity with histopathological subtypes and prognosis of gastric carcinoma. Ann Surg Oncol. 2002;9:887-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Corfield AP, Myerscough N, Longman R, Sylvester P, Arul S, Pignatelli M. Mucins and mucosal protection in the gastrointestinal tract: new prospects for mucins in the pathology of gastrointestinal disease. Gut. 2000;47:589-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 17. | Buisine MP, Devisme L, Savidge TC, Gespach C, Gosselin B, Porchet N, Aubert JP. Mucin gene expression in human embryonic and fetal intestine. Gut. 1998;43:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Reis CA, David L, Carvalho F, Mandel U, de Bolós C, Mirgorodskaya E, Clausen H, Sobrinho-Simões M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000;48:377-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Wang RQ, Fang DC, Liu WW, Men RP. Expression of MUC6 apomucin in the tissues of precancerous lesion and gastric carcinoma and its significance. Acta Acad Med Millitaris Tertiae. 2001;23:9-11. [Cited in This Article: ] |
| 20. | Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Rashid A, Hamilton SR. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol. 2002;161:611-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Grace A, Butler D, Gallagher M, Al-Agha R, Xin Y, Leader M, Kay E. APC gene expression in gastric carcinoma: an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2002;10:221-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Sugai T, Inomata M, Uesugi N, Jiao YF, Endoh M, Orii S, Nakamura S. Analysis of mucin, p53 protein and Ki-67 expressions in gastric differentiated-type intramucosal neoplastic lesions obtained from endoscopic mucosal resection samples: a proposal for a new classification of intramucosal neoplastic lesions based on nuclear atypia. Pathol Int. 2004;54:425-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Sun A, Noriki S, Imamura Y, Fukuda M. Detection of cancer clones in human gastric adenoma by increased DNA-instability and other biomarkers. Eur J Histochem. 2003;47:111-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Pemberton L, Taylor-Papadimitriou J, Gendler SJ. Antibodies to the cytoplasmic domain of the MUC1 mucin show conservation throughout mammals. Biochem Biophys Res Commun. 1992;185:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |