Abstract
The National Institute for Health and Clinical Excellence (NICE) invited the manufacturer of bevacizumab (Roche Products) to submit evidence for the clinical and cost effectiveness of this drug for the treatment of patients with metastatic colorectal cancer (mCRC), as part of the Institute’s Single Technology Appraisal (STA) process. The School of Health and Related Research (ScHARR) at the University of Sheffield was commissioned to act as the Evidence Review Group (ERG). This paper provides a description of the company submission, the ERG review and NICE’s subsequent decisions.
The ERG produced a critical review of the evidence for the clinical and cost effectiveness of the technology provided within the manufacturer’s submission to NICE. The ERG also independently searched for relevant evidence and modified the manufacturer’s decision analytic model to examine the impact of altering some of the key assumptions.
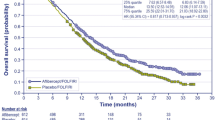
The main clinical effectiveness data were derived from a phase III, multicentre, multinational, two-arm, randomized, open-label study with the primary objective of confirming the non-inferiority of oxaliplatin plus capecitabine (XELOX) compared with oxaliplatin plus 5-fluorouracil and folinic acid (FOLFOX-4) in adult patients with histologically confirmed mCRC who had not previously been treated. The ERG considered that the NO 16966 trial was of reasonable methodological quality and demonstrated a significant improvement in both progression-free and overall survival when bevacizumab is added to either XELOX or FOLFOX-4. The ERG considered that the size of the actual treatment effect of bevacizumab was uncertain due to trial design limitations, imbalance of a known prognostic factor, relatively short treatment duration compared with that allowed within the trial protocol, and interpretation of the statistical analyses.
The manufacturer’s submission included a de novo economic evaluation using a cost-effectiveness model built in Microsoft® Excel. The ERG believed that the modelling structure employed was appropriate but highlighted several areas of uncertainty that had the potential to have a significant impact on the resulting incremental cost-effectiveness ratios (ICERs). The areas of uncertainty identified by the ERG included whether chemotherapy would be administered continuously or intermittently, patient access scheme (PAS) costs and uptake, survival that was dependent on the statistical analyses used, and the likely duration of continued treatment with bevacizumab after cessation of oxaliplatin and the efficacy associated with continuation.
The STA described here highlighted the challenges in appraising interventions with a complex PAS. Based on the analyses that include a discount to the list price of oxaliplatin, the ERG concluded that the ICERs for the addition of bevacizumab to XELOX or FOLFOX were both over £50 000. The NICE Appraisal Committee concluded that bevacizumab in combination with oxaliplatin and either 5-fluorouracil plus folinic acid or capecitabine (i.e. FOLFOX or XELOX) was not recommended for the treatment of mCRC.




Similar content being viewed by others
References
National Institute for Health and Clinical Excellence. Guide to the single technology appraisal (STA) process. London: NICE, 2006 Sep [online]. Available from URL: http://www.nice.org.uk/nicemedia/pdf/STA_Process_Guide.pdf [Accessed 2011 Oct 11]
Sculpher M. Single technology appraisal at the UK National Institute for Health and Clinical Excellence: a source of evidence and analysis for decision making internationally. Pharmacoeconomics 2010; 28(5): 347–9
Rodgers M, Griffin S, Paulden M, et al. Alitretinoin for severe chronic hand eczema: a NICE single technology appraisal. Pharmacoeconomics 2010; 28(5): 351–62
Bagust A, Greenhalgh J, Boland A, et al. Cetuximab for recurrent and/or metastatic squamous cell carcinoma of the head and neck: a NICE single technology appraisal. Pharmacoeconomics 2010; 28(6): 439–48
Stevenson M, Pandor A. Febuxostat for the management of hyperuricaemia in patients with gout: a NICE single technology appraisal. Pharmacoeconomics 2011; 29(2): 133–40
Scotland G, Waugh N, Royle P, et al. Denosumab for the prevention of osteoporotic fractures in post-menopausal women: a NICE single technology appraisal. Pharmacoeconomics 2011; 29(11): 951–61
Dickson R, Bagust A, Boland A, et al. Erlotinib monotherapy for the maintenance treatment of non-small cell lung cancer after previous platinum-containing chemotherapy: a NICE single technology appraisal. Pharmacoeconomics 2011; 29(12): 1051–62
McKenna C, Maund E, Sarowar M, et al. Dronedarone for the treatment of atrial fibrillation: a NICE single technology appraisal. Pharmacoeconomics 2012; 30(1): 35–46
Holmes M, Carroll C, Papaioannou D. Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a NICE single technology appraisal. Pharmacoeconomics 2012; 30(2): 137–46
Yang H, Craig D, Epstein D, et al. Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal. Pharmacoeconomics 2012; 30(4): 257–70
Boyers D, Jia X, Jenkinson D, et al. Eltrombopag for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a NICE single technology appraisal. Pharmacoeconomics 2012; 30(6): 483–95
Burch J, Griffin S, McKenna C, et al. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years: a NICE single technology appraisal. Pharmacoeconomics 2012; 30(11): 991–1004
Kilonzo M, Hislop J, Elders A. Pazopanib for the first-line treatment of patients with advanced and/or metastatic renal cell carcinoma: a NICE single technology appraisal. Pharmacoeconomics. In press
Craig D, Rice S, Paton F, et al. Retigabine for the adjunctive treatment of adults with partial onset seizures in epilepsy with and without secondary generalisation: a NICE single technology appraisal. Pharmacoeconomics. In press
Simpson EL, Fitzgerald P, Evans P, et al. Bivalirudin for the treatment of ST-segment elevation myocardial infarction: a NICE single technology appraisal. Pharmacoeconomics. In press
Armstrong N, Manuela J, van Asselt T, et al. Golimumab for the treatment of ankylosing spondylitis: a NICE single technology appraisal. Pharmacoeconomics. In press
Tosh J, Archer R, Davis S, et al. Golimumab for the treatment of rheumatoid arthritis after the failure of previous disease-modifying anti-rheumatic drugs: a NICE single technology appraisal. Pharmacoeconomics. In press
National Institute for Health and Clinical Excellence. Appraisal consultation document: bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer [online]. Available from URL: http://www.nice.org.uk/guidance/index.jsp?action=article&o=46346 [Accessed 2011 Oct 11]
National Institute for Health and Clinical Excellence. Final appraisal determination: bevacizumab in combination with oxaliplatin and either fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer [online]. Available from URL: http://www.nice.org.uk/nicemedia/live/12098/51510/51510.pdf [Accessed 2011 Oct 11]
Cancer Research UK. Bowel cancer statistics [online]. Available from URL: http://info.cancerresearchuk.org/cancerstats/types/bowel/ [Accessed 2012 Aug 27]
Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000; 355: 1041–7
de Gramont A, Figer A, Seymour MT, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18: 2938–47
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004; 22: 229–37
Nordlinger B, Van Cutsem E, Rougier P, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 2007; 43(14): 2037–45
National Institute for Health and Clinical Excellence. Irinotecan, oxaliplatin and raltitrexed for advanced colorectal cancer (review of TA33). NICE technology appraisal TA93. London: NICE, 2005 Aug [online]. Available from URL: http://guidance.nice.org.uk/TA93 [Accessed 2011 Oct 11]
National Institute for Health and Clinical Excellence. Capecitabine and tegafur uracil for metastatic colorectal cancer. NICE technology appraisal TA61. London: NICE, 2003 May [online]. Available from URL: http://guidance.nice.org.uk/TA61 [Accessed 2011 Oct 11]
National Institute for Health and Clinical Excellence. Cetuximab for the first-line treatment of metastatic colorectal cancer. NICE technology appraisal TA176. London: NICE, 2009 Aug [online]. Available from URL: http://guidance.nice.org.uk/ta176 [Accessed 2011 Oct 11]
National Institute for Health and Clinical Excellence. Bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. NICE technology appraisal TA118. London: NICE, 2007 Jan [online]. Available from URL: http://guidance.nice.org.uk/TA118 [Accessed 2011 Oct 11]
Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study [published errata appear in J Clin Oncol 2008 Jun; 26 (18): 3110 and J Clin Oncol 2009 Feb 1; 27 (4): 653]. J Clin Oncol 2008; 26(12): 2013–9
Giantonio BJ. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group study E3200. J Clin Oncol 2007; 25: 1539–44
O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol 2008; 10(26): 2336–41
European Medicines Agency (EMEA). Assessment report for avastin: international non-proprietary name/common name: bevacizumab. Procedure no. EMEA/H/C/000582/II/0014 [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000582/WC500029267.pdf [Accessed 2009 Sep 6]
Joint Formulary Committee. British National Formulary 57. London: BMJ Group and Pharmaceutical Press, 2009
Acknowledgements
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number 0821101) and will be published as part of a compendium of Evidence Review Group articles in Health Technology Assessment (HTA). See the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. This summary has not been externally peer reviewed by PharmacoEconomics.
The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
The authors have no competing interests.
Sophie Whyte undertook the critique of the economic evaluation and drafted the paper. Abdullah Pandor undertook the critique of the clinical effectiveness evidence presented, drafted the clinical effectiveness sections of the paper and suggested revisions. Matt Stevenson provided guidance throughout the STA and suggested revisions to the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Whyte, S., Pandor, A. & Stevenson, M. Bevacizumab for Metastatic Colorectal Cancer. PharmacoEconomics 30, 1119–1132 (2012). https://doi.org/10.2165/11597210-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11597210-000000000-00000