Abstract
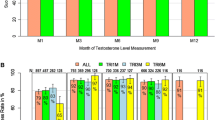
Background and Objectives: Triptorelin 6-month formulation was developed to offer greater convenience to both patients and physicians by reducing the injection frequency. The efficacy, pharmacokinetics and safety of a new 6-month formulation of triptorelin were investigated over 12 months (48 weeks). The primary objective was to evaluate the formulation in achieving castrate serum testosterone levels (≤1.735 nmol/L or ≤50 ng/dL) on day 29 and in maintaining castration at months 2–12. Absence of luteinizing hormone (LH) stimulation and change in prostate-specific antigen (PSA) level were also assessed.
Methods: An open-label, non-comparative, phase III study in 120 patients with advanced prostate cancer was conducted from July 2006 to August 2007 in private and public institutions in South Africa. Each patient received two consecutive intramuscular injections of triptorelin embonate (pamoate) 22.5 mg at an interval of 24 weeks. In all patients, testosterone (primary outcome measurement) was measured at baseline and then every 4 weeks; LH was measured before and 2 hours after the two injections. PSA was measured on day 1 and at weeks 12, 24, 36 and 48. Adverse events were recorded at each visit.
Results: In the intent-to-treat population, 97.5% (95% CI 92.9, 99.5) of patients achieved castrate serum testosterone levels by day 29, and 93.0% (95% CI 86.8, 97.0) maintained castration at months 2–12. After the second injection, 98.3% of patients showed absence of LH stimulation. The most frequent drug-related adverse events were hot flushes (71.7% of patients). No patient withdrew from the study as a result of an adverse event.
Conclusions: The triptorelin 6-month formulation was well tolerated and was able to achieve and maintain castration for the treatment of locally advanced and metastatic prostate cancer. By reducing the frequency of required injections, this new formulation offers a more convenient treatment regimen. (Clinical Trial Registration, NCT00751790 at www.clinicaltrials.gov)







Similar content being viewed by others
References
Palmberg C, Koivisto P, Visakorpi T, et al. PSA decline is an independent prognostic marker in hormonally treated prostate cancer. Eur Urol 1999; 36: 191–6
McLeod AG. Hormonal therapy: historical perspective to future directions. Urology 2003; 61: 3–7
Cassileth BR, Soloway MS, Vogelzang NJ, et al. Patient’s choice of treatment in stage D prostate cancer. Urology 1989; 33Suppl. 5: 57–62
Coy DH, Vilchez-Martinez JA, Coy EJ, et al. Analogs of luteinizing hormone-releasing hormone with increased biological activity produced by D-amino acid substitutions in position 6. J Med Chem 1976; 19: 423–5
Lahlou N. Pharmacokinetics and pharmacodynamics of triptorelin [in French]. Ann Urol (Paris) 2005 Oct; 39Suppl. 3: 78–84
Barron JL, Millar RP, Searle D. Metabolic clearance and plasma half-disappearance time of DTrp6 and exogenous luteinizing hormone-releasing hormone. J Clin Endocrinol Metab 1982; 54: 1169–73
Csernus VJ, Szende B, Schally AV. Release of peptides from sustained release delivery systems (microcapsules and microparticles) in vivo: a histological and immunohistochemical study. Int J Pept Protein Res 1990 Jun; 35(6): 557–65
National Comprehensive Cancer Network clinical practice guidelines in oncology: prostate cancer [online]. Available from URL: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp [Accessed 2009 Jun 16]
Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag, 2002
Wang C, Catlin DH, Demers LM, et al. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatographytandem mass spectrometry. J Clin Endocrinol Metab 2004; 89: 534–43
Sacks S. Are routine testosterone assays good enough? Clin Biochem Rev 2005; 26: 43–5
Clinical study report. Comparative testosterone pharmacodynamics and therapeutic efficacy of 1-month and 3-month formulations of triptorelin pamoate in patients with advanced prostate cancer, DEB-96-TRI-01 (first phase). Lausanne: Debiopharm SA, July 1999
Tornoe CW, Agerso H, Senderovitz T, et al. Population pharmacokinetic/pharmacodynamic (PK/PD) modeling of the hypothalamic-pituitary-gonadal axis following treatment with GnRH analogues. Br J Clin Pharmacol 2006; 63: 648–64
Smith MR. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res 2007; 13: 241–5
Tunn UW, Wiedey K. Safety and clinical efficacy of a new 6-month depot formulation of leuprorelin acetate in patients with prostate cancer in Europe. Prostate Cancer Prostatic Dis 2009; 12: 83–7
Commission de la transparence evaluation, Republique Francaise: Evaluation: Enantone LP (leuprorelin) 30 mg. Avis de transparence: Haute Autorité de Santé (HAS), St Denis de la Plaine; 2008 14 May
Crawford ED, Sartor O, Chu F, et al. A 12-month clinical study of LA-2585 (45.0mg): a new 6-month subcutaneous delivery system for leuprolide acetate for the treatment of prostate cancer. J Urol 2006; 175: 533–6
Acknowledgements
The study was sponsored by Debiopharm SA. Eija Lundström, Daniela Purcea, Pierre Grosgurin and Hervé Porchet are employees of Debiopharm SA. All the other authors received investigator fees for conduct of the study, but not for preparation of the paper, and have no conflicts of interest that are directly relevant to the content of this study.
The authors would like to thank Professor Per-Anders Abrahamsson for valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lundström, E.A., Rencken, R.K., van Wyk, J.H. et al. Triptorelin 6-Month Formulation in the Management of Patients with Locally Advanced and Metastatic Prostate Cancer. Clin. Drug Investig. 29, 757–765 (2009). https://doi.org/10.2165/11319690-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11319690-000000000-00000