Summary
The cure rate for children with acute lymphoblastic leukaemia (ALL) has increased to approximately 70%, in part related to the use of the protein synthesis inhibitor drug asparaginase in multiagent chemotherapy regimens. Its lack of haematological toxicity allows its incorporation into phases of therapy in which myelosuppression would be expected either from the disease itself (induction therapy) or secondary to other chemotherapeutic agents (consolidation, intensification or reinduction phases of therapy). Its antileukaemic effect is related to the degree and duration of asparagine depletion.
The 2 native forms of L-asparaginase are derived from Escherichia coli and Ewinia chrysantherni. The half-lives (t½) of these forms are approximately 1.2 and 0.6 days, respectively. In order to increase the biological t½, pegaspargase was synthesised by the covalent attachment of monomethoxypolyethylene glycol (PEG) to native E. coli L-asparaginase: it has a t½, of approximately 5.7 days. The duration of asparagine depletion, the substrate amino acid of the drug, is directly related to asparaginase t½.
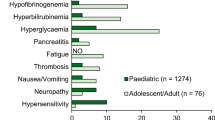
Asparaginase is associated with several unique toxicities, including hyperglycaemia, hypolipoproteinaemia, hypoalbuminaemia, coagulation factor deficiencies, hepatotoxicity and pancreatitis. Since asparaginase is a protein, it may induce hypersensitivity reactions. The incidence of these reactions increases with use. In addition, silent hypersensitivity, i.e. the development of IgG antibodies without clinical reactions, results in a decreased t½ of asparaginase, shortened duration of asparagine depletion, and probably decreased efficacy. The use of pegaspargase allows continued treatment with asparaginase in patients with clinical hypersensitivity reactions. In addition, its use in patients with silent hypersensitivity may maintain the efficacy of asparaginase.
So far, the optimal use of the 3 forms of asparaginase has not been determined in children with ALL, partly due to the lack of appropriate pharmacokinetic monitoring methods. As the technology has become available, it has been demonstrated that there is little rationale for the dosage and administration schedules presently in use. Studies are required to determine appropriate dosages and administration methods (intravenous or intramuscular) and schedules for each form of asparaginase, based upon pharmacokinetic parameters. The incidence and time to onset of hypersensitivity (clinical or silent) reactions and the appropriate means of continuing asparaginase therapy with therapeutic effect needs to be evaluated. Pharmacokinetic studies are now available as a research tool. These will allow further investigation to determine if failure to maintain asparagine depletion is a remediable cause of treatment failure.
Similar content being viewed by others
References
Capizzi RL, Holcenberg JS. Asparaginase. In: Holland JF, Frei E III, Bast Jr RC, et al., editors. Cancer medicine. Philadelphia: Lea & Febiger, 1993: 796–805
Nesbit ME, Ertel I, Hammond GD. L-Asparaginase as a single agent in acute lymphocytic leukemia: survey of studies from Childrens Cancer Study Group. Cancer Treat Rep 1981; 65: 101–7
Carbone PP, Haskell CM, Leventhal BG, et al. Clinical experience with L-asparaginase. Recent Results Cancer Res 1970; 33: 236–43
Beard MEJ, Crowther D, Galton DAG, et al. L-Asparaginase in treatment of acute leukaemia and lymphosarcoma. BMJ 1970; 1: 191–5
Ohnuma T, Rosner F, Levy RN, et al. Treatment of adult leukemia with L-asparaginase (NSC-109229). Cancer Chemother Rep 1971; 55: 269–75
Hill JM, Loeb E, MacLellan A, et al. Response to highly purified L-asparaginase during therapy of acute leukemia. Cancer Res 1969; 29: 1574–80
Abshire T, Pollock B, Billet A, et al. Weekly polyethylene glycol conjugated (PEG) L-asparaginase (ASP) produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia (rALL): a Pediatric Oncology Group (POG) Study 9310 [abstract]. Proc Am Soc Clin Oncol 1995; 14: 344
Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. I. Course of transplanted cancers of various kinds in mice and rats given guinea pig serum, horse serum, or rabbit serum. J Exp Med 1953; 98: 565–82
Kidd JG. Regression of transplanted lymphomas induced in vivo by means of normal guinea pig serum. II. Studies on the nature of the active serum constituent: histological mechanism of the regression: tests for effects of guinea pig serum on lymphoma cells in vitro: discussion. J Exp Med 1953; 98: 583–606
Broome JD. Evidence that the L-asparaginase activity of guinea pig serum is responsible for its antilymphoma effects. Nature 1961; 191: 1114–5
Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. I. Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J Exp Med 1963; 118: 99–120
Broome JD. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects. II. Lymphoma 6C3HED cells cultured in a medium devoid of L-asparagine lose their susceptibility to the effects of guinea pig serum in vivo. J Exp Med 1963; 118: 121–48
Broome JD. Studies on the mechanism of tumor inhibition by L-asparaginase. J Exp Med 1968; 127; 1055–72
Mashburn LT, Wriston Jr JC. Tumor inhibitory effect of Lasparaginase from Escherichia coli. Arch Biochem Biophys 1964; 105: 450–2
Miller HK, Salser JS, Balis ME. Amino acid levels following L-asparagine amidohydrolase (EC.3.5.1.1) therapy. Cancer Res 1969; 29: 183–7
Jackson RC, Handschumacher RE. Escherichia coli L-asparaginase: catalytic activity and subunit nature. Biochemistry 1970; 9: 3585–90
Bonthron DT. L-Asparaginase II of Escherichia coli K-12: cloning, mapping and sequencing of the ansβ gene. Gene 1990; 91: 101–5
Oettgen HF, Old LL, Boyse EA, et al. Inhibition of leukemias in man by L-asparaginase. Cancer Res 1967; 27: 2619–31
Hill JM, Roberts J, Loeb E, et al. L-Asparaginase therapy for leukemia and other malignant neoplasms. JAMA 1967; 202: 882–8
Hill JM, Loeb E, MacLellan A, et al. Response to highly purified L-asparaginase during therapy of acute leukemia. Cancer Res 1979; 29: 1574–80
Tallal L, Tan C, Oettgen H, et al. E. coli L-asparaginase in the treatment of leukemia and solid tumors in 131 children. Cancer 1970; 25: 306–20
Jones B, Holland JF, Glidewell O, et al. Optimal use of L-asparaginase (NSC-109229) in acute lymphocytic leukemia. Med Pediatr Oncol 1977; 3: 387–400
Ertel IJ, Nesbit ME, Hammond D, et al. Effective dose of L-asparaginase for induction of remission in previously treated children with acute lymphocytic leukemia: a report from Childrens Cancer Study Group. Cancer Res 1979; 39: 3893–6
Nesbit ME, Ertel I, Hammond GD. L-Asparaginase as a single agent in acute lymphocytic leukemia: survey of studies from Childrens Cancer Study Group. Cancer Treat Rep 1981; 65: 101–7
Nesbit M, Chard R, Evans A, et al. Evaluation of intramuscular versus intravenous administration of L-asparaginase in childhood leukemia. Am J Pediatr Hematol Oncol 1979; 1: 9–13
Sallan SE, Hitchcock-Bryan S, Gelber R, et al. Influence of intensive asparaginase in the treatment of childhood non-T cell acute lymphoblastic leukemia. Cancer Res 1983; 43: 5601–7
Clavell LA, Gelber RD, Cohen HJ, et al. Four-agent induction and intensive asparaginase therapy for treatment of childhood acute lymphoblastic leukemia. N Engl J Med 1986; 315: 657–63
Desai SJ, Barr RD, Andrew M, et al. Management of Ontario children with acute lymphoblastic leukemia by the Dana-Farber Cancer Institute protocols. Can Med Assoc J 1989; 141: 693–7
Evans WE, Tsiatis A, Rivera G, et al. Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer 1982; 49: 1378–83
Fabry U, Korholz D, Jurgens H, et al. Anaphylaxis to L-asparaginase during treatment for acute lymphoblastic leukemia in children: evidence of a complement-mediated mechanism. Pediatr Res 1985; 19: 400–8
Weiss RB. Hypersensitivity reactions. Semin Oncol 1992; 19: 458–77
Park YK, Abuchowski A, Davis S, et al. Pharmacology of Escherichia coli L-asparaginase polyethylene glycol adduct. Anticancer Res 1981; 1: 373–6
Kurtzberg J, Friedman H, Asselin B, et al. The use of polyethylene glycol-conjugated L-asparaginase (PEG-ASP) in pediatric patients with prior hypersensitivity to native L-asparaginase [abstract]. Proc Am Soc Clin Oncol 1990; 9: 219
Kurtzberg J, Asselin B, Pollack B, et al. PEG-L-asparaginase (PEGasp) vs native E coli asparaginase (asp) for reinduction of relapsed acute lymphoblastic leukemia (ALL): POG #8866 phase II trial [abstract]. Proc Am Soc Clin Oncol 1993; 12: 325
Ettinger LJ, Kurtzberg J, Voute PA, et al. An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer 1995; 75: 1176–81
Wade H, Elsworth R, Herbert D, et al. A new L-asparaginase with antitumor activity. Lancet 1968; II: 776–7
Eden OB, Shaw MP, Lilleyman JS, et al. Non-randomized study comparing toxicity of Escherichia coli and Erwinia asparaginase in children with leukaemia. Med Pediatr Oncol 1990; 18: 497–502
Billet AL, Carls A, Gelber RD, et al. Allergic reactions to Erwinia asparaginase in children with acute lymphoblastic leukemia who had previous allergic reactions to Escherichia coli asparaginase. Cancer 1992; 70: 201–6
Barron AC, Luke KH, Hsy E, et al. The use of Erwinia L-asparaginase as first line therapy for childhood acute lymphocytic leukemia. Int J Pediatr Hematol/Oncol 1995; 2: 7–10
Asselin B, Gelber R, Sallan S. Relative toxicity of E. coli L-asparaginase (ASP) and pegaspargase (PEG) in newly diagnosed childhood acute lymphoblastic leukemia (ALL) [abstract]. Blood 1995; 86: 177a
Cheung NKV, Chau IY, Coccia PF. Antibody response to Escherichia coli L-asparaginase. Am J Pediatr Hematol Oncol 1986: 8: 99–104
Kurtzberg J, Asselin B, Poplack D, et al. Antibodies to asparaginases alter pharmacokinetics and decrease enzyme activity in patients on asparaginase therapy [abstract]. Proc Am Assoc Cancer Res 1993; 34: 304
Asselin BL, Whitin JC, Coppola DJ, et al. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol 1993; 11: 1780–6
Kurtzberg J. A new look at PEG-L-asparaginase and other asparaginases in hematological malignancies [abstract]. Cancer Invest 1994; 12Suppl. 1: 59–60
Kurtzberg J, Asselin B, Poplack D, et al. PEG-L-asparaginase (PEG-ASP) pharmacology in pediatric patients with acute lymphoblastic leukemia (ALL) [abstract]. Proc Am Soc Clin Oncol 1994; 13: 144
Werber G, Boos J, Ahlke E, et al. Pharmacokinetic/pharmacodynamic monitoring of L-asparaginase in children [abstract]. In: Abstracts of the International Symposium Acute Leucemias VI: Prognostic Factors and Treatment Strategies. Ann Hematol 1995 Suppl. II; 70: 130
Rizzari C, Gentili D, D’Incaici M, et al. Inadequate L-asparagine (L-ASN) depletion after repeated courses of Erwinia L-asparaginase (L-ASP) administration in children with acute lymphoblastic leukemia (ALL). Med Pediatr Oncol 1995; 25: 282
Riccardi R, Holcenberg JS, Glaubiger DL, et al. L-Asparaginase pharmacokinetics and asparagine levels in cerebrospinal fluid of rhesus monkeys and humans. Cancer Res 1981; 41: 4554–8
Dibenedetto SP, Di Cataldo A, Ragusa R, et al. Levels of L-asparagine in CSF after intramuscular administration of asparaginase from Erwinia in children with acute lymphoblastic leukemia. J Clin Oncol 1995; 13: 339–44
Peters BG, Gowckner BJ, Ponzillo JJ, et al. Economic assessment: pegaspargase versus asparaginase in acute lymphocytic leukemia. Formulary 1995; 30: 388–93
Author information
Authors and Affiliations
Corresponding author
Additional information
1 A review from the Children’s Cancer Group Asparaginase Task Force.
Rights and permissions
About this article
Cite this article
Ettinger, L.J., Ettinger, A.G., Avramis, W.I. et al. Acute Lymphoblastic Leukaemia. BioDrugs 7, 30–39 (1997). https://doi.org/10.2165/00063030-199707010-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00063030-199707010-00005