Abstract
Background and objective
Lanreotide is a somatostatin analogue used for the treatment of acromegaly and neuroendocrine tumours. The objective of this study was to develop a pharmacokinetic model for the sustainedrelease formulation lanreotide Autogel® after deep subcutaneous administration in healthy subjects, and to explore the potential effect of covariates, especially sex and dose.
Subjects and methods
This was an open-label, single-centre, randomized, dose-ranging, parallel-group study, with a follow-up period of 4–7 months following drug administration in healthy subjects. Healthy Caucasian subjects aged 18–45 years were included. Subjects received a rapid intravenous bolus of 7 μg/kg of an immediate-release formulation of lanreotide (lanreotide IRF). After a 3-day washout period, participants were randomized to receive a single deep subcutaneous injection of lanreotide Autogel® at a dose of 60, 90 or 120 mg.
Pharmacokinetic and statistical analysis
Blood samples for lanreotide determination were obtained during the first 12 hours after the intravenous bolus injection and during the 4- to 7-month follow-up period after deep subcutaneous administration of lanreotide Autogel®. Data after intravenous and subcutaneous administration were fitted simultaneously using the population approach in NONMEM® version VI software. The model was validated externally using data from patients with acromegaly.
Results
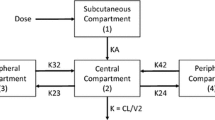
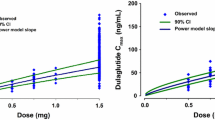
In total, 50 healthy subjects (24 women and 26 men) received a single intravenous dose of lanreotide IRF. Of these, 38 subjects (18 women and 20 men) received a single subcutaneous dose of lanreotide Autogel® 3 days after intravenous lanreotide IRF. The disposition of lanreotide was described by a three-compartment open model. The estimates of the total volume of distribution and serum clearance were 15.1 L and 23.1 L/h, respectively. The estimates of interindividual variability were <40%. To evaluate lanreotide Autogel® pharmacokinetics, the absorption rate was modelled to decrease exponentially as a function of the natural logarithm of time. The absolute bioavailability after deep subcutaneous administration of lanreotide Autogel® was 63%. The rate of absorption and bioavailability of lanreotide Autogel® were independent of the administered dose in the range from 60 to 120 mg, and no significant effect of covariates (sex, dose, age or bodyweight) was found (p > 0.05).
Conclusions
Population analysis allows a full description of the disposition of lanreotide after rapid intravenous bolus administration of lanreotide IRF (7 μg/kg) and the pharmacokinetics of lanreotide Autogel® after a single deep subcutaneous injection (60, 90 or 120 mg) in healthy subjects. The model-based simulations provide support for the feasibility of extending the dosing interval for lanreotide Autogel® to 56 days when given at 120 mg. The absorption profile of lanreotide Autogel® was independent of the dose and was not affected by sex.












Similar content being viewed by others
References
Giusti M, Ciccarelli E, Dallabonzana D, et al. Clinical results of long-term slowrelease lanreotide treatment of acromegaly. Eur J Clin Invest 1997; 27: 277–84
Cannavò S, Squadrito S, Curtò L, et al. Results of a two-year treatment with slow release lanreotide in acromegaly. Horm Metab Res 2000; 32(6): 224–9
Lightman S. Somatuline Autogel: an extended release lanreotide formulation. Hosp Med 2002; 63(3): 162–5
Bronstein M, Musolino N, Jallad R, et al. Pharmacokinetic profile of lanreotide Autogel® in patients with acromegaly after four deep subcutaneous injections of 60, 90 or 120 mg every 28 days. Clin Endocrinol 2005; 63: 514–9
Caron P, Beckers A, Cullen DR, et al. Efficacy of the new long-acting formulation of lanreotide (lanreotide Autogel) in the management of acromegaly. J Clin Endocrinol Metab 2002; 87(1): 99–104
Caron P, Bex M, Cullen DR, et al. One-year follow-up of patients with acromegaly treated with fixed or titrated doses of lanreotide Autogel. Clin Endocrinol (Oxf) 2004; 60(6): 734–40
Rubin J, Ajani J, Schirmer W, et al. Octreotide acetate long-acting formulation versus open-label subcutaneous octreotide acetate in malignant carcinoid syndrome. J Clin Oncol 1999; 17(2): 600–6
Cendrós J, Peraire C, Trocóniz I, et al. Pharmacokinetics and population pharmacodynamic analysis of lanreotide Autogel. Metabolism 2005; 54: 1276–81
Barbanoj M, Antonijoan R, Morte A, et al. Pharmacokinetics of the somatostatin analog lanreotide in patients with severe chronic renal insufficiency. Clin Pharmacol Ther 1999; 66(5): 485–91
Vahl N, Moller N, Lauritzen T, et al. Metabolic effects and pharmacokinetics of a growth hormone pulse in healthy adults: relation to age, sex, and body composition. J Clin Endocrinol Metab 1997; 82: 3612–8
NONMEM® user’s guides. Beal SL, Sheiner LB, Boeckmann AJ, editors. Ellicott City (MA): Icon Development Solutions, 1989-2006
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001; 28: 481–504
Karlsson MO. Handling data below the limit of quantification (BQL) — including simultaneous modeling of continuous and categorical data [lecture]. NONMEM® VI Workshop; 2008 Mar 17–19; San Francisco (CA)
Ludden TM, Beal SL, Sheiner LB. Comparison of the Akaike Information Criterion, the Schwarz Criterion and the F test as guides to model selection. J Pharmacokinet Biopharm 1994; 22: 431–45
Holford N, Ambros R, Stoeckel K. Models for describing absorption rate and estimating extent of bioavailability: application to cefetamet pivoxil. J Pharmacokin Biopharm 1992; 20: 421–42
Piotrovskii VK. The use of Weibull distribution to describe the in vivo absorption kinetics. J Pharmacokinet Biopharm 1987; 15: 681–6
Savic RM, Jonker DM, Kerbusch T, et al. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 2007; 34: 711–26
Holford N. The visual predictive check: superiority to standard diagnostic (Rorschach) plots [abstract no. 738]. 14th Meeting, Population Approach Group in Europe; 2005 Jun 16–17; Pamplona [online]. Available from URL: http://www.page-meeting.org/default.asp?abstract=738 [Accessed 2008 Oct 6]
Ramis J, Cendrós J-M, Catus F, et al. Pharmacokinetics and pharmacodynamics of lanreotide Autogel® after multiple subcutaneous administration in patients with acromegaly: a 52-week, multi-centre, randomized study [poster]. 12th International Congress of Endocrinology; 2004 Aug 31–Sep 4; Lisbon
Antonijoan RM, Barbanoj MJ, Cordero JA, et al. Pharmacokinetics of a new Autogel formulation of the somatostatin analogue lanreotide after a single subcutaneous dose in healthy volunteers. J Pharm Pharmacol 2004; 56(4): 471–6
Rowland M, Tozer T. Clinical pharmacokinetics: concepts and applications. 3rd ed. Philadelphia (PA): Williams & Wilkins, 1995
Valery C, Paternostre M, Robert B, et al. Biomimetic organization: octapeptide self-assembly into nanotubes of viral capsid-like dimension. Proc Natl Acad Sci U S A 2003; 100(18): 10258–62
Valery C, Artzner F, Robert B, et al. Self-association process of a peptide in solution: from beta-sheet filaments to large embedded nanotubes. Biophys J 2004; 86(4): 2484–501
Keller A, Wu Z, Kratzsch J, et al. Pharmacokinetics and pharmacodynamics of GH: dependence on route and dosage of administration. Eur J Endocrinol 2007; 156: 647–53
Lucas T, Astorga R. Efficacy of lanreotide Autogel administered every 4–8 weeks in patients with acromegaly previously responsive to lanreotide microparticles, 30 mg: a phase III trial. J Clin Endocrinol Metab 2006; 65: 320–6
Acknowledgements
The clinical study in healthy subjects was designed by Ipsen Pharma S.p.A. and the Institute for Pharmacokinetics and Analytical Studies (IPAS), conducted at the IPAS clinic (Ligornetto, Switzerland) and coordinated by Ipsen. All pharmacokinetics-related activities, including assays of lanreotide plasma concentrations and pharmacokinetic analyses, were performed by Ipsen in collaboration with University of Navarra. Maria Garrido and Iñaki Trocóniz have received research funding from Ipsen. Josep-María Cendrós, Concepción Peraire, Joaquim Ramis and Paolo Boscani are employed by Ipsen. The manuscript was prepared by Rosendo Obach, an employee of Ipsen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trocóniz, I.F., Cendrós, JM., Peraire, C. et al. Population Pharmacokinetic Analysis of Lanreotide Autogel® in Healthy Subjects. Clin Pharmacokinet 48, 51–62 (2009). https://doi.org/10.2165/0003088-200948010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/0003088-200948010-00004