Summary
Synopsis
Olanzapine is a thienobenzodiazepine derivative which displays efficacy in patients with schizophrenia and related psychoses. It has structural and pharmacological properties resembling those of the atypical antipsychotic clozapine and an improved tolerability profile compared with the classical antipsychotic haloperidol.
In several large, well controlled trials in patients with schizophrenia or related psychoses, olanzapine generally 5 to 20 mg/day was at least as effective as haloperidol (5 to 20mg) and more so than placebo, as assessed by overall rating scales for psychoses. Olanzapine improved negative symptoms to a greater extent than haloperidol in 2 of 3 comparative trials, including the largest trial. Efficacy of olanzapine has a rapid onset (within 1 to 2 weeks). Its clinical benefits appear to be maintained for treatment periods of up to I year, as shown by analysis of the extension phase of several trials demonstrating decreased probability of hospitalisation over this period, compared with haloperidol. Preliminary data suggest the drug may also improve quality of life.
Olanzapine was associated with significantly fewer adverse movement disorders (e.g. akathisia, dystonia, hypertonia, extrapyramidal symptoms) than haloperidol. There have been no reports of agranulocytosis (as occurs with clozapine) or any other haemotoxicity attributed to olanzapine, and the drug has shown minimal effect on prolactin levels. Transient increases in levels of hepatic transaminases seem to be clinically unimportant. The only events recorded more frequently during olanzapine than during haloperidol therapy were weight gain, dry mouth and increased appetite.
Although the antipsychotic activity of olanzapine has been well demonstrated, its efficacy in refractory schizophrenia and its place relative to other atypical antipsychotic s remain to be determined. Nevertheless, if the long term tolerability profile of olanzapine is confirmed, the drug should provide a valuable therapeutic alternative in the management of schizophrenia and related psychoses.
Pharmacodynamic Properties
Olanzapine is a thienobenzodiazepine derivative with affinity for a number of neurotransmitter receptors. It has significant in vitro inhibitory activity at dopamine D1, D2, D4, serotonin (5-hydroxytryptamine; 5-HT) 5-HT2A, 5-HT2C, histamine H1, α1-adrenergic and muscarinic receptors. The mixed receptor activity of olanzapine is similar to that of clozapine. The in vitro binding affinity of olanzapine, like that of clozapine, is greater for 5-HT2 receptors than for dopamine D2 receptors.
In electrophysiological studies, olanzapine produced a differential effect on nigrostriatal and mesolimbic systems within the CNS, which may be predictive of a low potential for the induction of extrapyramidal symptoms (EPS).
Olanzapine is active in many animal behavioural models predictive of antipsychotic activity. Olanzapine also inhibited a number of dopamine and serotonin agonist-induced behaviours in vivo, confirming in vitro evidence of its receptor affinity profile. In animal models considered predictive of the potential to induce EPS, most studies indicate that olanzapine has less propensity than classical anti-psychotics to induce these effects.
In patients with schizophrenia, olanzapine produced minimal effects on prolactin levels. Increases seen in some patients appear to be transient and smaller than those produced by haloperidol.
Pharmacokinetic Properties
As shown in healthy volunteers, maximum plasma olanzapine concentrations (Cmax) after single oral doses (2.5 to 12.2mg) are reached within about 5 hours. Values for Cmax and area under the plasma concentration-time curve are proportional to the dose. The volume of distribution of olanzapine is large (reported to range from 10.3 to 18.4 L/kg).
Olanzapine is extensively metabolised: at least 10 different metabolites, which appear to be inactive, have been identified. The elimination half-life (t1/2β) of olanzapine ranged from 27 to 38.6 hours in young healthy individuals. Elderly and female volunteers have shown a decreased total body clearance and a prolonged t1/2β.
Although the metabolism of olanzapine is mediated, at least in part, by cytochrome P450 enzymes, there appears to be little potential for olanzapine to interact with other drugs metabolised by these enzymes.
Therapeutic Efficacy
Olanzapine is more effective than placebo and at least as effective as haloperidol, as shown in a number of large, randomised, double-blind trials in patients with schizophrenia and related psychoses. Indeed, in 2 of 3 comparative trials, including the largest, some significant differences were evident between olanzapine and haloperidol. Reductions in BPRS total scores with olanzapine tended to be dose dependent. Mean reductions in BPRS total scores ranged from 16 to 39% for patients receiving olanzapine 2.5 to 17.5 mg/day, 0.5 to 8% for placebo groups and were about 30% in those given haloperidol 10 to 20 mg/day. Significant differences between olanzapine ≥7.5 mg/day and placebo were evident within 1 to 2 weeks, indicating a relatively rapid onset of effect.
Olanzapine ameliorates both the positive and negative symptoms of schizophrenia and appears to produce greater improvements than haloperidol in the latter. In 2 comparisons, olanzapine was associated with significantly greater reductions than placebo in the BPRS-positive subscale (23 to 33% vs 0% and 12%) and the BPRS-negative subscale (20 to 41% vs 3% and 6%). In some, but not all, assessments of subscales for negative symptoms, olanzapine in dosages >7.5 mg/day was more effective than haloperidol 5 to 20 mg/day, as measured by the BPRS-negative subscale and the SANS scale. The largest trial demonstrated a significant difference between the 2 drugs as shown by the negative subscales of the PANSS and BPRS scales. According to a path analysis, olanzapine appears to exert a direct influence on primary as well as secondary negative symptoms.
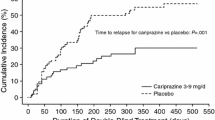
Pooled data from the extension phases of randomised, controlled trials revealed that olanzapine 2.5 to 20 mg/day for up to 1 year was associated with a higher probability of maintaining a response (defined as the absence of hospitalisation for psychosis) than either placebo or haloperidol (5 to 20mg). This suggests that the efficacy of olanzapine is maintained over this treatment period. Preliminary data indicate that olanzapine may improve quality of life to a greater extent than haloperidol.
Tolerability
In placebo-controlled trials, the only adverse events occurring more often with olanzapine than with placebo were somnolence (12 to 39%), constipation (6 to 15%) and weight gain (0 to 12%). When compared with haloperidol, olanzapine was associated with significantly fewer adverse movement disorders, tremor, nervousness and salivation but a higher frequency of weight gain, dry mouth and increased appetite.
Olanzapine demonstrated little potential for the induction of EPS, as measured by rating scales assessing parkinsonism, akathisia and dystonia. Olanzapine produced either no effect on or small improvements in rating scale scores and was associated with significantly lower scores than haloperidol. The incidence of treatment-emergent dyskinetic effects was significantly lower with olanzapine than with haloperidol, as shown by combined analysis of 3 long term trials in 1155 patients. Using the AMDP-5 scale, olanzapine was associated with a significantly higher incidence of 2 items (excessive appetite and dry mouth) compared with 25 for haloperidol.
Treatment with olanzapine causes occasional elevations in levels of hepatic transaminases; however, the increases appear to be transient and no clinical evidence of hepatotoxicity has been documented. No significant effect on haematological parameters (e.g. agranulocytosis) has been reported in patients receiving olanzapine, including those who had previously experienced clozapine-induced dyscrasias.
Dosage and Administration
The recommended dosage of olanzapine is 5 to 1 Omg once daily initially, without regard for meals, aiming to reach a dosage of 10 mg/day within several days. Dosage may be adjusted using 5mg increments at intervals of at least 1 week. Dosages ≥15 mg/day should be implemented only after clinical assessment. The tolerability of dosages of 20 mg/day has not been assessed.
A starting dosage of 5 mg/day is used in patients who are debilitated or predisposed to hypotension, or who may exhibit slower olanzapine metabolism (women aged ≥65 years who are nonsmokers). Responding patients should receive maintenance treatment using the lowest effective dose.
Olanzapine should be used with caution in patients with, or at risk of, the following conditions: cardiovascular, cerebrovascular or other disease which may predispose to the development of hypotension; hepatic dysfunction; and seizures. Because somnolence may occur with olanzapine, patients should use caution if operating machinery or driving.
Similar content being viewed by others
References
Meltzer HY, Fibiger HC. Olanzapine: a new atypical antipsychotic drug. Neuropsychopharmacology 1996 Feb; 14: 83–5
Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996 Feb; 14: 87–96
Schotte A, Janssen PFM, Gommeren W, et al. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 1996 Mar; 124: 57–73
Roth BL, Craigo SC, Choudhary MS, et al. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther 1994; 268(3): 1403–10
Meltzer HY. Pre-clinical pharmacology of atypical antipsychotic drugs: a selective review. Br J Psychiatry 1996 May; 168 Suppl. 29: 23–31
Roth BL, Tandra S, Burgess LH, et al. D4 dopamine receptor binding affinity does not distinguish between typical and atypical antipsychotic drugs. Psychopharmacology 1995 Aug; 120(3): 365–8
Seeman P, Van Tol HMM. Dopamine receptor pharmacology. Curr Opin Neurol Neurosurg 1993; 6: 602–8
Murray AM, Hyde TN, Knable MB, et al. Distribution of putative D4 dopamine receptors in postmorten striatum from patients with schizophrenia. J Neurosci 1995; 15: 2186–91
Seeman P, Guan H-C, Van Tol HHM. Dopamine D4 receptors elevated in schizophrenia. Nature 1993; 365: 441–5
Meltzer HY, Matsubara S, Lee J-C. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin3 pKi values. J Pharmacol Exp Ther 1989; 251: 238–46
Robertson GS, Fibiger HC. Effects of olanzapine on regional C-Fos expression in rat forebrain. Neuropsychopharmacology 1996 Feb; 14: 105–10
Pilowsky LS, Busatto GF, Taylor M, et al. Dopamine D2 receptor occupancy in vivo by the novel atypical antipsychotic olanzapine - a 12311BZM single photon emission tomography (SPET) study. Psychopharmacology 1996 Mar; 124: 148–53
Nyberg S, Farde L, Halidin C. A PET study of 5-HT2 and D2 dopamine receptor occupancy induced by olanzapine in healthy subjects. Eli Lilly and Company (Data on file)
Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: a critical analysis. Psychopharmacology 1996; 124: 2–34
Stockton ME, Rasmussen K. Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology 1996 Feb; 14: 97–104
Skarsfeldt T. Differential effects of repeated administration of novel antipsychotic drugs on the activity of midbrain dopamine neurons in the rat. Eur J Pharmacol 1995 Aug 15; 281: 289–94
Stockton ME, Rasmussen K. Olanzapine, a novel atypical antipsychotic, reverses d-amphetamine-induced inhibition of midbrain dopamine cells. Psychopharmacology 1996 Mar; 124: 50–6
Bymaster FP, Hemrick-Luecke SK, Perry KW, et al. Neuro-chemical evidence for antagonism by olanzapine of dopamine, serotonin, α1-adrenergic and muscarinic receptors in vivo in rats. Psychopharmacology 1996 Mar; 124: 87–94
Corbett R, Camacho F, Woods AT, et al. Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology 1995 Jul; 120(1): 67–74
Moore NA, Tye NC, Axton MS, et al. The behavioral pharmacology of olanzapine, a novel atypical antipsychotic agent. J Pharmacol Exp Ther 1992 Aug; 262: 545–51
Deveney AM, Waddington JL. Comparison of the new atypical antipsychotics olanzapine and ICI 204,636 with clozapine on behavioural responses to the selective D1-like dopamine receptor agonist A 68930 and selective D2-like agonist RU 24213. Psychopharmacology 1996 Mar; 124: 40–9
Hoffman DC, Donovan H. Catalepsy as a rodent model for detecting antipsychotic drugs with extrapyramidal side effect liability. Psychopharmacology 1995 Jul; 120(2): 128–33
Gosselin O, Majchrzak M, Oberling P, et al. Restoration of the amphetamine-disrupted latent inhibition by olanzapine in the rat. Behav Pharmacol 1996; 7 Suppl. 1: 46
Bakshi VP, Geyer MA. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology 1995 Nov; 122(2): 198–201
Das S, Fowler SC. Similarity of clozapine’s and olanzapine’s acute effects on rats’ lapping behavior. Psychopharmacology 1996 Feb; 123(4): 374–8
Benvenga MJ, Leander JD. Olanzapine, an atypical antipsychotic, increases rates of punished responding in pigeons. Psychopharmacology 1995 May; 119: 133–8
Moore NA, Rees G, Sanger G, et al. Effects of olanzapine and other antipsychotic agents on responding maintained by a conflict schedule. Behav Pharmacol 1994 Apr; 5: 196–202
Nanry KP, Pollard GT, Howard JL. Olanzapine moderately increases conflict responding but does not produce a benzodiazepine-like cue in rat. Drug Dev Res 1995 Mar; 34: 317–9
Cools AR, Prinssen EPM, Ellenbroek BA. The olfactory tubercle as a site of action of neuroleptics with an atypical profile in the paw test: effect of risperidone, prothipendyl, ORG 5222, sertindole and olanzapine. Psychopharmacology 1995 Jun; 119: 428–39
Skarsfeldt T. Differential effect of antipsychotics on place navigation of rats in the Morris water maze: a comparative study between novel and reference antipsychotics. Psychopharmacology 1996 Mar; 124: 126–33
Beasley Jr CM, Tollefson G, Tran P. Olanzapine versus placebo and haloperidol: acute phase results of the North American double-blind olanzapine trial. Neuropsychopharmacology 1996 Feb; 14: 111–23
Tran P, Beasley C, Street J, et al. Olanzapine versus haloperidol: acute results of the multi-center international trial [poster]. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Tran P, Beasley C, Tollefson G, et al. Acute and long-term results of the dose ranging double-blind olanzapine trial [poster]. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Fuller RW, Snoddy HD. Neuroendocrine evidence for antagonism of serotonin and dopamine receptors by olanzapine (LY170053), an antipsychotic drug candidate. Res Commun Chem Pathol Pharmacol 1992 Jul; 77: 87–93
Boyd D, Obermeyer BD, Nyhart Jr EH, et al. The disposition of olanzapine in healthy volunteers [abstract no. 120]. Pharmacologist 1993; 35(3): 176
Bergstrom RF, Callaghan JT, Cerimele BJ, et al. Pharmacokinetics of olanzapine in elderly and young [abstract]. Pharm Res 1995 Sep; 12 Suppl.: S358
Patel BR, Nyhart Jr EH, Callaghan JT, et al. Combined population pharmacokinetic analysis of olanzapine in healthy volunteers [abstract]. Pharm Res 1995 Sep; 12 Suppl.: 360
Kassahun K, Mattiuz EL, Gillespie TA, et al. Disposition and biotransformation of [14C]olanzapine, a new antipsychotic agent, in humans labstract]. ISSX Proc 1994; 6: 100
Ring BJ, Catlow J, Lindsay TJ, et al. Identification of the human cytochromes P450 responsible for the in vitro formation of the major oxidative metabolites of the antipsychotic agent olanzapine. J Pharmacol Exp Ther 1996 Feb; 276: 658–66
Ring BJ, Binkley SN, Vandenbranden M, et al. In vitro interaction of the antipsychotic agent olanzapine with human cytochromes P450 CYP2C9, CYP2C19, CYP2D6 and CYP3A. Br J Clin Pharmacol 1996 Mar; 41: 181–6
Demolie D, Onkelinx C, Müller-Oerlinghausen B. Interaction between olanzapine and lithium in healthy male volunteers [abstract no. 4861. Therapie 1995; 50 Suppl.
Awad AG, Voruganti LNP, Heslegrave RJ. The aims of antipsychotic medication: what are they and are they being achieved? CNS Drugs 1995; 4(1): 8–16
Guy W. ECDEU Assessment manual for psychopharmacology, revised version. Bethesda, MD, US Department of Health, Education and Welfare, 1976
Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 1988; 24: 112–7
Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Multi-Health Systems, North Tonawanda, New York, 1996
Andreason NC. Negative symptoms in schizophrenia. Arch Gen Psychiatry 1982; 39: 784–8
Beasley Jr CM, Sanger T, Satterlee W, et al. Olanzapine versus placebo: results of a double-blind, fixed dose olanzapine trial. Psychopharmacology 1996 Mar; 124: 159–67
American Psychiatric Association Task Force on Nomenclature and Statistics. Diagnostic and statistical manual of mental disorders. 3rd ed., revised. Washington, DC, APA Press, 1987
Tollefson GD, Beasley Jr CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia, schizoaffective and schizophreniform disorders: results of an international collaborative trial. Eli Lilly and Company (Data on file)
Baldwin DS, Montgomery SA. First clinical experience with olanzapine (LY 170053): results of an open-label safety and dose-ranging study in patients with schizophrenia. Int Clin Psychopharmacol 1995 Nov; 10: 239–44
Ishigooka J, Miura S, Murasaki M, et al. Clinical efficacy and safety of olanzapine in Japanese schizophrenics [abstract no. P-7-7J. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia: 59
Tollefson G, Lu Y. Comorbid mood disturbance in schizophrenia [poster]. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Tollefson G, Sanger TM, Beasley CM. The course of primary and secondary negative symptoms in a controlled trial with olanzapine [poster]. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Satterlee WG, Dellva MA, Beasley CM, et al. Effectiveness of olanzapine in long-term continuation treatment [poster]. 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Martin C, Genduso L, Revicki D, et al. Quality of life outcomes of olanzapine, a new atypical antipsychotic agent (abstract). Presented at the Winter Workshop on Schizophrenia; 1996 Mar, Crans-Montana, Switzerland
Revicki D, Genduso LA, Hamilton SL, et al. Quality of life outcomes for olanzapine and haloperidol treatment for schizophrenia and related psychotic disorders (abstract). Presented at the American Psychiatry Association Annual Meeting; 1996, New York, USA
Martin C, Genduso L, Revicki D, et al. Quality of life and olanzapine treatment in schizophrenia: results of an international double-blind randomized clinical trial (abstract). Presented at the Winter Workshop on Schizophrenia; 1996 Mar, Crans-Montana, Switzerland
Beasley C, Tollefson G, Wood A, et al. Safety overview of olanzapine (abstract). Presented at the X World Congress of Psychiatry; 1996 Aug, Madrid, Spain
Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 1970 Suppl. 212: 11–9
Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154: 672–6
Street J, Tamura R, Sanger T, et al. Long-term treatment-emergent dyskinetic symptoms in patients treated with olanzapine and haloperidol. Eur Neuropsychopharmacol 1996 Jun; 6 Suppl. 3: 132
Olanzapine prescribing information. Eli Lilly and Company, Indianapolis, USA, 1996
Wagstaff AJ, Bryson HM. Clozapine: a review of its pharmacological properties and therapeutic use in patients with schizophrenia who are unresponsive to or intolerant of classical antipsychotic agents. CNS Drugs 1995 Nov; 4(5): 370–400
Tollefson GD. Olanzapine: a novel drug candidate for the treatment of schizophrenia (abstract). 20th Congress of the International College of Neuropsychopharmacology (CINP); 1996 Jun 23–27; Melbourne, Australia
Terkelsen KG, Menikoff A. Measuring the costs of schizophrenia: implications for the post-institutional era in the US. Pharmaco Economics 1995; 8(3): 199–222
Fleischhacker WW. New drugs for the treatment of schizophrenic patients. Acta-Psychiatrica-Scandinavica 1995; 91(388) Suppl.: 24–30
Kerwin R, Taylor D. New antipsychotics. Drug Ther 1996 Jul; 6(1): 71–82
Abbott Serlect schizophrenia seven-arm study could meet FDA standards for comparative labeling; Serlect will compete against Lilly’s Zyprexa. FDC Rep Pink Sheet 1996 November: 13–6
Hummer M, Fleischhacker WW. Compliance and outcome in patients treated with antipsychotics: the impact of extrapyramidal syndromes. CNS Drugs 1996; 5 Suppl. 1: 13–20
Lilly-funded MEDSTAT schizophrenia care and assessment program to build disease management database; Zyprexa/Risperdal study to conclude by December. FDC Rep Pink Sheet 1996 Oct 7: 7
Möller H-J. Neuroleptic treatment of negative symptoms in schizophrenic patients. Efficacy problems and methodological difficulties. Eur Neuropsychopharmacol 1993 Mar; 3: 1–11
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: A.G. Awad, Clarke Institute of Psychiatry, Toronto, Ontario, Canada; D. Baldwin, Department of Psychiatry, University of Southampton, Royal South Hants Hospital, Southampton, England; R.L. Borison, Department of Psychiatry, Medical College of Georgia, Augusta, Georgia, USA; A. Farmer, Division of Psychological Medicine, University of Wales College of Medicine, Cardiff, Wales; S.C. Fowler, Human Development and Family Life, The University of Kansas, Lawrence, Kansas, USA; J. Gerlach, Institute of Biological Psychiatry, St Hans Hospital, Roskilde, Denmark; J. Ishigooka, Department of Psychiatry, Kitasato University School of Medicine, Sagamihara, Japan; R. Kerwin, Department of Psychological Medicine, Institute of Psychiatry, London, England; T. Silverstone, London, England.
Rights and permissions
About this article
Cite this article
Fulton, B., Goa, K.L. Olanzapine. Drugs 53, 281–298 (1997). https://doi.org/10.2165/00003495-199753020-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199753020-00007