Abstract
Purpose
Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor, has shown increased pathological complete response rates when added to neoadjuvant chemotherapy. In various cancer types, bevacizumab treatment was accompanied by an increased risk of bleedings and other surgical complications. We assessed associated surgical complications.
Methods
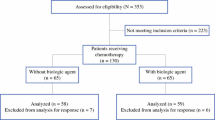
In the GeparQuinto trial, 1,948 patients were randomized to receive four cycles epirubicin/cyclophosphamide (EC, 90/600 mg/m2 q3w) followed by four cycles docetaxel (D, 100 mg/m2 q3w) each with (ECB-DB) or without (EC-D) bevacizumab (B, 15 mg/kg q3w) concurrent with chemotherapy. Surgery had to be performed not earlier than 28 days after the last bevacizumab infusion, but within days 21 and 35 after the last chemotherapy.
Results
In 743 (38.1 %) patients, a surgical complication (bleedings, hematomas, necrosis, wound infections, abscess) was documented prospectively. Baseline characteristics of the patients were well balanced between both arms. The breast-conserving surgery (BCS) rate (N = 502) was 69.1 % (EC-D) and 71.9 % (ECB-DB; p = 0.464). The first surgical procedure was performed at a median of 29 (EC-D) and 34 days (ECB-DB) after last chemotherapy with or without bevacizumab infusion (p < 0.001). Surgical complications were documented in 38 (10.9 %; EC-D) and 59 (15.0 %; ECB-DB) patients (p = 0.103). Surgical complications were significantly higher after ECD-DB only in patients treated with BCS (N = 53; p = 0.029) or in those requiring repeat surgery in order achieve clear margins (N = 23; p = 0.037) compared to the EC-D group.
Conclusions
Addition of bevacizumab to neoadjuvant chemotherapy might be associated with an increased risk for surgical complications in patients treated with BCS or after repeated surgeries.

Similar content being viewed by others
References
Chang HY, Nuyten DS, Sneddon JB, et al. Robustness, scalability, and integration of a wound-response gene expression signature in predicting breast cancer survival. Proc Natl Acad Sci USA. 2005;102:3738–43.
von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309.
Gerber B, Loibl S, Eidtmann H, et al. Neoadjuvant bevacizumab and anthrcycline-taxane based chemotherapy in 678 triple negative primary breast cancers; results from the GEPARQUINTO study (GBG 44). Ann Oncol. 2013;24(12):2978–84. doi:10.1093/annonc/mdt361.
Makhoul I, Klimberg VS, Korourian S, et al. Combined neoadjuvant chemotherapy with bevacizumab improves pathologic complete response in patients with hormone receptor negative operable or locally advanced breast cancer. Am J Clin Oncol. 2013. doi:10.1097/COC.0b013e31828940c3.
Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30:2039–45.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11.
Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–44.
Scappaticci FA, Fehrenbacher L, Cartwright T, et al. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J Surg Oncol. 2005;91:173–80.
Kabbinavar FF, Schulz J, McCleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–705.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Reck M, Barlesi F, Crinò L, et al. Predicting and managing the risk of pulmonary haemorrhage in patients with NSCLC treated with bevacizumab: a consensus report from a panel of experts. Ann Oncol. 2012;23:1111–20.
Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–34.
Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:20–30.
Smith IE, Pierga JY, Biganzoli L, et al. First-line bevacizumab plus taxane-based chemotherapy for locally recurrent or metastatic breast cancer: safety and efficacy in an open-label study in 2,251 patients. Ann Oncol. 2011;22:595–602.
Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–77.
Kriegel I, Cottu PH, Fourchotte V, et al. Wound healing and catheter thrombosis after implantable venous access device placement in 266 breast cancers treated with bevacizumab therapy. Anticancer Drugs. 2011;22:1020–3.
Cottu PH, Fourchotte V, Vincent-Salomon A, Kriegel I, Fromantin I. Necrosis in breast cancer patients with skin metastases receiving bevacizumab-based therapy. J Wound Care. 2011;20:403–4, 406, 408.
Lecarpentier E, Ouaffi L, Mir O, et al. Bevacizumab-induced small bowel perforation in a patient with breast cancer without intraabdominal metastases. Invest New Drugs. 2011;29:1500–3.
Lazzati V, Zygon J, Lohsiriwat V, Veronesi P, Petit JY. Impaired wound healing and bilateral mastectomy flap necrosis in a patient with locally advanced breast cancer after neoadjuvant Paclitaxel with bevacizumab. Aesthetic Plast Surg. 2010;34:796–7.
Kube R, Meyer F, Bien N, et al. Surgical management of bevacizumab-associated peritonitis due to perforation. Zentralbl Chir. 2009;134:462–7.
Kim HR, Jung KH, Im SA, et al. Multicentre phase II trial of bevacizumab combined with docetaxel-carboplatin for the neoadjuvant treatment of triple-negative breast cancer (KCSG BR-0905). Ann Oncol. 2013;24:1485–90.
Li CY, Shan S, Huang Q, et al. Initial stages of tumor cell–induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–7.
Huober J, Fasching PA, Hanusch C, et al. Neoadjuvant chemotherapy with paclitaxel and everolimus in breast cancer patients with non-responsive tumours to epirubicin/cyclophosphamide (EC) +/− bevacizumab—results of the randomised GeparQuinto study (GBG 44). Eur J Cancer. 2013;49:2284–93.
Bear HD, Tang G, Rastogi P, et al. The effect on surgical complications of bevacizumab added to neoadjuvant chemotherapy: NSABP protocol B-40. Cancer Res. 2011. doi:10.1158/0008-5472.SABCS11-PD07-08.
Golshan M, Garber JE, Gelman R, et al. Does neoadjuvant bevacizumab increase surgical complications in breast surgery? Ann Surg Oncol. 2011;18:733–7.
Gordon CR, Rojavin Y, Patel M, et al. A review on bevacizumab and surgical wound healing: an important warning to all surgeons. Ann Plast Surg. 2009;62:707–9.
Erinjeri JP, Fong AJ, Kemeny NE, Brown KT, Getrajdman GI, Solomon SB. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2011;117:1296–301.
Cortes J, Caralt M, Delaloge S, et al. Safety of bevacizumab in metastatic breast cancer patients undergoing surgery. Eur J Cancer. 2012;48:475–81.
NCI. Common Terminology Criteria for Adverse Events (CTCAE) and Common Toxicity Criteria (CTC). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 5 Nov 2013.
Acknowledgment
We thank all patients and investigators for participation and Sanofi-Aventis and Roche, Germany, for financial support of this trial (NCT 00567554).
Disclosure
Gunter von Minckwitz has received honoraria and research found from Roche. Holger Eidtmann has received personal fees from Roche. Claus Hanusch has received personal fees from Novartis, Amgen, and Boeringer Ingelheim. Jens Blohmer has received honoraria from Roche. Jens Huober has received personal fees from Roche and Sanofi Aventis. Sibylle Loibl has received honoraria and research found from Roche. The other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the AGO B and the German Breast Group Investigators
Rights and permissions
About this article
Cite this article
Gerber, B., von Minckwitz, G., Eidtmann, H. et al. Surgical Outcome after Neoadjuvant Chemotherapy and Bevacizumab: Results from the GeparQuinto Study (GBG 44). Ann Surg Oncol 21, 2517–2524 (2014). https://doi.org/10.1245/s10434-014-3606-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-014-3606-9