Abstract
Background
The prognostic role of circulating tumor cells (CTCs) has been established for colorectal cancer (CRC). We investigated the qualitative and quantitative detection of CTC in the central (CVBC) and mesenteric (MVBC) venous blood compartments to elucidate the patterns of hematogenous tumor cell dissemination in patients with CRC.
Methods
A total of 200 patients were enrolled prospectively. Blood samples were collected from the tumor-draining vein and via a central venous line. CTCs were detected and quantified by using the CellSearch system. Factors associated with CTC detection in both compartments were analyzed by using univariate and multivariate analyses.
Results
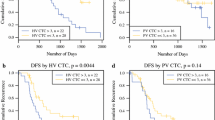
CTC analyses were performed in the CVBC and MVBC in 200 and 80 patients, respectively. CTCs were found at a higher rate (P = 0.01) and at a higher count (P = 0.006) in the MVBC compared with the CVBC. On multivariate analyses, stage IV disease (odds ratio, 3.83; 95% confidence interval, 1.42–10.35) and increased preoperative carbohydrate antigen 19–9 level (odds ratio, 3.57; 1.30–9.79) were associated with CTC detection in the CVBC. CTCs were detected more frequently (P = 0.05) and at higher numbers (P = 0.05) in the CVBC of patients with low compared with mid or high rectal tumors.
Conclusions
The qualitative and quantitative detection of CTCs is higher in the MVBC compared with the CVBC of patients with CRC.

Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet. 2005;365(9454):153–65.
Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–56.
Colombano SP, Reese PA. The cascade theory of metastatic spread: are there generalizing sites? Cancer. 1980;46:2312–4.
Makrilia N, Kollias A, Manolopoulos L, et al. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–37.
Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410(6824):50–6.
Koch M, Weitz J, Kienle P, et al. Comparative analysis of tumor cell dissemination in mesenteric, central, and peripheral venous blood in patients with CRC. Arch Surg. 2001;136:85–9.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–8.
Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic CRC. J Clin Oncol. 2008;26:3213–21.
Sobin LH, Gospodarowicz MK, Wittekind C. UICC: TNM classification of malignant tumours. New York: Wiley & Sons; 2009.
Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904.
Nakamori S, Kameyama M, Furukawa H, et al. Genetic detection of CRC cells in circulation and lymph nodes. Dis Colon Rectum. 1997;40(10 Suppl):S29–36.
Taniguchi T, Makino M, Suzuki K, et al. Prognostic significance of reverse transcriptase-polymerase chain reaction measurement of carcinoembryonic antigen mRNA levels in tumor drainage blood and peripheral blood of patients with colorectal carcinoma. Cancer. 2000;89:970–6.
Yamaguchi K, Takagi Y, Aoki S, et al. Significant detection of circulating cancer cells in the blood by reverse transcriptase-polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232:58–65.
Fujita S, Kudo N, Akasu T, et al. Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis. 2001;16:141–6.
Sadahiro S, Suzuki T, Tokunaga N, et al. Detection of tumor cells in the portal and peripheral blood of patients with colorectal carcinoma using competitive reverse transcriptase-polymerase chain reaction. Cancer. 2001;92:1251–8.
Iinuma H, Okinaga K, Egami H, et al. Usefulness and clinical significance of quantitative real-time RT-PCR to detect isolated tumor cells in the peripheral blood and tumor drainage blood of patients with colorectal cancer. Int J Oncol. 2006;28:297–306.
Shafik A, Mostafa H. Study of the arterial pattern of the rectum and its clinical application. Acta Anat. 1996;157:80–6.
Sugarbaker PH. Metastatic inefficiency: the scientific basis for resection of liver metastases from colorectal cancer. J Surg Oncol Suppl. 1993;3:158–60.
Mizuno N, Kato Y, Izumi Y, et al. Importance of hepatic first-pass removal in metastasis of colon carcinoma cells. J Hepatol. 1998;28:865–77.
Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–8.
Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82.
Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–17.
Klein CA, Blankenstein TJ, Schmidt-Kittler O, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet 2002;360:683–9.
Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010;467:1109–13.
Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–26.
O’Connor ES, Greenblatt DY, Loconte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388.
Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9.
Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat Cell Genet. 1979;5:957–71.
Magnani JL, Brockhaus M, Smith DF, et al. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981;212(4490):55–6.
Eche N, Pichon MF, Quillien V, et al. [Standards, options and recommendations for tumor markers in colorectal cancer]. Bull Cancer. 2001;88:1177–206.
Yakabe T, Nakafusa Y, Sumi K, et al. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol. 2010;17:2349–56.
Nakagoe T, Sawai T, Tsuji T, et al. Preoperative serum level of CA19-9 predicts recurrence after curative surgery in node-negative colorectal cancer patients. Hepatogastroenterology. 2003;50:696–9.
Herszenyi L, Farinati F, Cardin R, et al. Tumor marker utility and prognostic relevance of cathepsin B, cathepsin L, urokinase-type plasminogen activator, plasminogen activator inhibitor type-1, CEA and CA 19-9 in colorectal cancer. BMC Cancer. 2008;8: 194.
Park IJ, Choi GS, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29:4303–8.
Svobodova S, Topolcan O, Holubec L Jr, et al. Parameters of biological activity in colorectal cancer. Anticancer Res. 2011;31:373–8.
Dabelsteen E. Cell surface carbohydrates as prognostic markers in human carcinomas. J Pathol. 1996;179:358–69.
Acknowledgment
This work was supported by the Deutsche Forschungsgemeinschaft (WE 3548/4-1).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Rahbari and Dr. Bork contributed equally to this work.
Rights and permissions
About this article
Cite this article
Rahbari, N.N., Bork, U., Kircher, A. et al. Compartmental Differences of Circulating Tumor Cells in Colorectal Cancer. Ann Surg Oncol 19, 2195–2202 (2012). https://doi.org/10.1245/s10434-011-2178-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-011-2178-1