Abstract
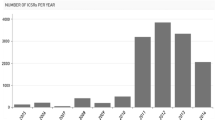
The main aim of the WHO International Drug Monitoring Programme, started in 1968, is to identify the earliest possible pharmacovigilance signals. The program now has more than 80 member countries from all parts of the world contributing individual case safety reports (ICSRs) to the WHO Global ICSR Database System, VigiBase.
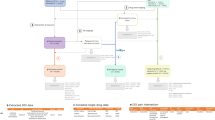
VigiBase is maintained and developed on behalf of WHO by the Uppsala Monitoring Centre (UMC), situated in Uppsala, Sweden. The database system includes the ICH E2B compatible ICSR database, the WHO Drug Dictionaries (WHO-DD and -DDE), and the medical terminologies WHO Adverse Reaction Terminology (WHO-ART), International Classification of Diseases (ICD), and the Medical Dictionary for Regulatory Activities (MedDRA).
Apart from data management and quality assurance tools, the VigiBase system includes a web-based reporting tool, an automated signal detection process using advanced data mining, and search facilities, available to the member countries and, on request, to other stakeholders.
Similar content being viewed by others
References
Begaud B, et al. Rates of spontaneous reporting of adverse drug reactions in France. JAMA. 2002; 288: 1588.
Lindquist M. Seeing and Observing in International Pharmacovigilance—Achievements and Prospects in Worldwide Drug Safety. Nijmegen: University of Nijmegen; 2003.
Rawlins MD. Pharmacovigilance: paradise lost, regained or postponed? The William Withering Lecture 1994. J R Coll Physicians Lond. 1995;29:41–49.
Lindquist M, et al. A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Safety. 2000;23:533–542.
Stahl M, et al. Assessing the impact of drug safety signals from the WHO database presented in “SIGNAL”: results from a questionnaire of national pharmacovigilance centres. Drug Safety. 2003;26: 721–727.
Norén GN, et al. Extending the methods used to screen the WHO drug safety database towards analysis of complex associations and improved accuracy for rare events. Stat Med. 2006;25: 3740–3757.
Norén GN, Orre R, Bate A, Edwards IR. Duplicate detection in adverse drug reaction surveillance. Data Mining Knowledge Discovery. 2007;14:305–328.
Further Reading
Bate A. The Use of a Bayesian Confidence Propagation Neural Network in Pharmacovigilance. Umeå University; 2003.
Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54: 315–321.
Bate A, Lindquist M, Edwards IR, Orre R. A data mining approach for signal detection and analysis. Drug Safety. 2002;25:393–397.
Bate A, Lindquist M, Orre R, Edwards IR, Meyboom RH. Data-mining analyses of pharmacovigilance signals in relation to relevant comparison drugs. Eur J Clin Pharmacol. 2002;58:483–490.
Bate A, Orre R, Lindquist M, Edwards IR. Explanation of data mining methods. BMJ. 2001. http://www.bmj.com/cgi/content/full/322/7296/1207/DC1/cgi/content/full/322/7296/1207/DC1.
Bate A, Orre R, Lindquist M, Edwards IR. Pattern recognition using a recurrent neural network and its application to the WHO database. Pharmacoepidemiol Drug Safety. 2001;10:S163 (abstract).
Bate AJ, Lindquist M, Edwards IR, Orre R. Understanding quantitative signal detection methods in spontaneously reported data. Pharmacoepidemiol Drug Safety. 2002;11:S214–S215.
CIOMS. Harmonization of data fields for electronic transmission of case-report information internationally. Geneva: CIOMS; 1995: 48.
Edwards IR. The accelerating need for pharmacovigilance. J R Coll Physicians Lond. 2000;34:48–51.
Edwards IR. Pharmacovigilance—beyond 2000. Reactions. 2000;(783):3–5.
Edwards IR, Fucik H. Impact and credibility of the WHO adverse reaction signals. Drug Inf J. 1996; 30:461–464.
Edwards IR, Lindquist M, Wiholm BE, Napke E. Quality criteria for early signals of possible adverse drug reactions. Lancet. 1990;336:156–158.
Edwards IR, Olsson S. The WHO International Drug Monitoring Programme. In: Aronson JK, ed. Side Effects of Drugs, Annual 25: Elsevier Science B.V., 2002: 589–598.
Farah MH, Edwards IR, Lindquist M. Key issues in herbal pharmacovigilance. Adverse Drug Reactions J. 2000;2:105–109.
Finney DJ. Monitoring adverse reactions to drugs— its logic and its weaknesses. Proc Eur Soc Study Drug Toxicity. 1966:198–207.
Finney DJ. Systematic signalling of adverse reactions to drugs. Methods Inf Med. 1974;13:1–10.
Fucik H, Backlund A, Farah M. Building a computerized herbal substance register for implementation and use in the World Health Organisation international drug monitoring programme. Drug Inf J. 2002;36:839–854.
Lindquist M. Data quality management in pharmacovigilance. Drug Safety. 2004;27:857–870.
Lindquist M. The WHO Programme for International Drug Monitoring: the present and future. In: Mitchard M, ed. Electronic Communication Technologies. Buffalo: Interpharm Press; 1998: 527–549.
Lindquist M, Edwards IR, Bate A, Fucik H, Nunes AM, Ståhl M. From association to alert—a revised approach to international signal analysis. Pharmacoepidemiol Drug Safety. 1999;8:S15–S25.
Lindquist M, Farah MH, Edwards R. Monitoring of herbal medicines. Clin Pharmacol Ther. 2000:18.
Lindquist M, Pettersson M, Edwards IR, et al. Omeprazole and visual disorders: seeing alternatives. Pharmacoepidemiol Drug Safety. 1996; 5:27– 32.
Lindquist M, Pettersson M, Edwards IR, et al. How does cystitis affect a comparative risk profile of tiaprofenic acid with other non-steroidal antiin-flammatory drugs? An international study based on spontaneous reports and drug usage data. ADR Signals Analysis Project (ASAP) Team. Pharmacol Toxicol. 1997;80:211–217.
Lindquist M, Sanderson J, Claesson C, Imbs JL, Rohan A, Edwards IR. New pharmacovigilance information on an old drug—an international study of spontaneous reports on digoxin. Drug Invest. 1994;8:73–80.
Orre R, Lansner A, Bate A, Lindquist M. Bayesian neural networks with confidence estimations applied to data mining. Computational Stat Data Anal. 2000;34:473–493.
Ståhl M, Lindquist M, Edwards IR, Brown EG. Introducing triage logic as a new strategy for the detection of signals in the WHO Drug Monitoring Database. Drug Safety. 2003;26:721–727.
Uppsala Monitoring Centre. http://www.who-umc.org.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lindquist, M. VigiBase, the WHO Global ICSR Database System: Basic Facts. Ther Innov Regul Sci 42, 409–419 (2008). https://doi.org/10.1177/009286150804200501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1177/009286150804200501