Abstract
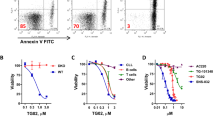
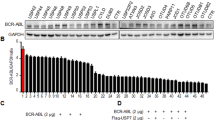
Recently, proteasome inhibitors (PI) have attracted interest as novel anticancer agents in B-cell chronic lymphocytic leukemia (B-CLL). A prominent feature of B-CLL cells is the high expression of CD23, which is closely related to cell survival and is regulated by Notch2. Since several components of the Notch signaling cascade are tightly regulated by proteasomal degradation, we studied the effect of PI on Notch2 activity and CD23 expression. Exposure of B-CLL cells to PI led to induction of apoptosis, a time- and dose-dependent downregulation of CD23 expression and a decline in DNA binding of transcriptionally active Notch2. In contrast, the transcription factor NF-AT and its putative target gene CD5, which is highly expressed in B-CLL cells, were unaffected. When the late phase of PI-induced apoptosis was arrested by inhibition of caspase 3, the reduction of Notch2 activity was still observed, indicating that reduction of active Notch2 took place already during an earlier phase of apoptosis. Enforced expression of constitutively active Notch2 decreased PI-mediated apoptosis in a human B-cell line. These data indicate that downregulation of CD23 and loss of Notch2 activity are early steps in PI-induced apoptosis of B-CLL lymphocytes and may be part of the full apoptotic response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kay NE, Hamblin TJ, Jelinek DF, Dewald GW, Byrd JC, Farag S et al. Chronic lymphocytic leukemia. Hematology (Am Soc Hematol Educ Program) 2002; 175: 193–213.
Guipaud O, Deriano L, Salin H, Vallat L, Sabatier L, Merle-Beral H et al. B-cell chronic lymphocytic leukaemia: a polymorphic family unified by genomic features. Lancet Oncol 2003; 4: 505–514.
Jewell AP . Role of apoptosis in the pathogenesis of B-cell chronic lymphocytic leukaemia. Br J Biomed Sci 2002; 59: 235–238.
Fournier S, Yang LP, Delespesse G, Rubio M, Biron G, Sarfati M . The two CD23 isoforms display differential regulation in chronic lymphocytic leukaemia. Br J Haematol 1995; 89: 373–379.
Hubmann R, Schwarzmeier JD, Shehata M, Hilgarth M, Duechler M, Dettke M et al. Notch2 is involved in the overexpression of CD23 in B-cell chronic lymphocytic leukemia. Blood 2002; 99: 3742–3747.
Artavanis-Tsakonas S, Rand MD, Lake RJ . Notch signaling: cell fate control and signal integration in development. Science 1999; 284: 770–776.
Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A . Signalling downstream of activated mammalian Notch. Nature 1995; 377: 355–358.
Schroeter EH, Kisslinger JA, Kopan R . Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998; 393: 382–386.
Berezovska O, Jack C, McLean P, Aster JC, Hicks C, Xia W et al. Rapid Notch1 nuclear translocation after ligand binding depends on presenilin-associated gamma-secretase activity. Ann N Y Acad Sci 2000; 920: 223–226.
Ellisen LW, Bird J, West DC, Soreng AL, Reynolds TC, Smith SD et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 1991; 66: 649–661.
Capobianco AJ, Zagouras P, Blaumueller CM, Artavanis-Tsakonas S, Bishop JM . Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol Cell Biol 1997; 17: 6265–6273.
Witt CM, Hurez V, Swindle CS, Hamada Y, Klug CA . Activated Notch2 potentiates CD8 lineage maturation and promotes the selective development of B1 B cells. Mol Cell Biol 2003; 23: 8637–8650.
Bertrand FE, Eckfeldt CE, Lysholm AS, LeBien TW . Notch-1 and Notch-2 exhibit unique patterns of expression in human B-lineage cells. Leukemia 2000; 14: 2095–2102.
Witt CM, Won WJ, Hurez V, Klug CA . Notch2 haploinsufficiency results in diminished B1 B cells and a severe reduction in marginal zone B cells. J Immunol 2003; 171: 2783–2788.
Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 2003; 18: 675–685.
Deftos ML, He YW, Ojala EW, Bevan MJ . Correlating notch signaling with thymocyte maturation. Immunity 1998; 9: 777–786.
Jehn BM, Bielke W, Pear WS, Osborne BA . Cutting edge: protective effects of notch-1 on TCR-induced apoptosis. J Immunol 1999; 162: 635–638.
Miele L, Osborne B . Arbiter of differentiation and death: notch signaling meets apoptosis. J Cell Physiol 1999; 181: 393–409.
Shelly LL, Fuchs C, Miele L . Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem 1999; 73: 164–175.
Sade H, Krishna S, Sarin A . The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem 2004; 279: 2937–2944.
Qiu L, Joazeiro C, Fang N, Wang HY, Elly C, Altman Y et al. Recognition and ubiquitination of Notch by Itch, a hect-type E3 ubiquitin ligase. J Biol Chem 2000; 275: 35734–35737.
Wu G, Lyapina S, Das I, Li J, Gurney M, Pauley A et al. SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol Cell Biol 2001; 21: 7403–7415.
Oberg C, Li J, Pauley A, Wolf E, Gurney M, Lendahl U . The Notch intracellular domain is ubiquitinated and negatively regulated by the mammalian Sel-10 homolog. J Biol Chem 2001; 276: 35847–35853.
Jehn BM, Dittert I, Beyer S, von der MK, Bielke W . c-Cbl binding and ubiquitin-dependent lysosomal degradation of membrane-associated Notch1. J Biol Chem 2002; 277: 8033–8040.
Naujokat C, Hoffmann S . Role and function of the 26S proteasome in proliferation and apoptosis. Lab Invest 2002; 82: 965–980.
Ciechanover A . The ubiquitin proteolytic system and pathogenesis of human diseases: a novel platform for mechanism-based drug targeting. Biochem Soc Trans 2003; 31: 474–481.
Adams J . Proteasome inhibitors as new anticancer drugs. Curr Opin Oncol 2002; 14: 628–634.
Gillessen S, Groettup M, Cerny T . The proteasome, a new target for cancer therapy. Onkologie 2002; 25: 534–539.
Masdehors P, Merle-Beral H, Magdelenat H, Delic J . Ubiquitin–proteasome system and increased sensitivity of B-CLL lymphocytes to apoptotic death activation. Leuk Lymphoma 2000; 38: 499–504.
Schenkein D . Proteasome inhibitors in the treatment of B-cell malignancies. Clin Lymphoma 2002; 3: 49–55.
Pahler JC, Ruiz S, Niemer I, Calvert LR, Andreeff M, Keating M et al. Effects of the proteasome inhibitor, bortezomib, on apoptosis in isolated lymphocytes obtained from patients with chronic lymphocytic leukemia. Clin Cancer Res 2003; 9: 4570–4577.
Wong SC, Chew WK, Tan JE, Melendez AJ, Francis F, Lam KP . Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. J Biol Chem 2002; 277: 30707–30715.
Lenz HJ . Clinical update: proteasome inhibitors in solid tumors. Cancer Treat Rev 2003; 29 (Suppl 1): 41–48.
Magill L, Walker B, Irvine AE . The proteasome: a novel therapeutic target in haematopoietic malignancy. Hematology 2003; 8: 275–283.
Masdehors P, Omura S, Merle-Beral H, Mentz F, Cosset JM, Dumont J et al. Increased sensitivity of CLL-derived lymphocytes to apoptotic death activation by the proteasome-specific inhibitor lactacystin. Br J Haematol 1999; 105: 752–757.
Ling PD, Hsieh JJ, Ruf IK, Rawlins DR, Hayward SD . EBNA-2 upregulation of Epstein–Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J Virol 1994; 68: 5375–5383.
Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch1. Mol Cell 2000; 5: 197–206.
Das I, Craig C, Funahashi Y, Jung KM, Kim TW, Byers R et al. Notch oncoproteins depend on gamma-secretase/prencilin activity for processing and function. J Biol Chem 2004; 279: 30771–30780.
Schuh K, Avots A, Tony HP, Serfling E, Kneitz C . Nuclear NF-ATp is a hallmark of unstimulated B cells from B-CLL patients. Leuk Lymphoma 1996; 23: 583–592.
Berland R, Wortis HH . An NFAT-dependent enhancer is necessary for anti-IgM-mediated induction of murine CD5 expression in primary splenic B cells. J Immunol 1998; 161: 277–285.
Adams J . Preclinical and clinical evaluation of proteasome inhibitor PS-341 for the treatment of cancer. Curr Opin Chem Biol 2002; 6: 493–500.
Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM . Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene 2003; 22: 2643–2654.
Almond JB, Snowden RT, Hunter A, Dinsdale D, Cain K, Cohen GM . Proteasome inhibitor-induced apoptosis of B-chronic lymphocytic leukaemia cells involves cytochrome c release and caspase activation, accompanied by formation of an approximately 700 kDa Apaf-1 containing apoptosome complex. Leukemia 2001; 15: 1388–1397.
Jesenberger V, Jentsch S . Deadly encounter: ubiquitin meets apoptosis. Nat Rev Mol Cell Biol 2002; 3: 112–121.
Masdehors P, Merle-Beral H, Maloum K, Omura S, Magdelenat H, Delic J . Deregulation of the ubiquitin system and p53 proteolysis modify the apoptotic response in B-CLL lymphocytes. Blood 2000; 96: 269–274.
Rangarajan A, Syal R, Selvarajah S, Chakrabarti O, Sarin A, Krishna S . Activated Notch1 signaling cooperates with papillomavirus oncogenes in transformation and generates resistance to apoptosis on matrix withdrawal through PKB/Akt. Virology 2001; 286: 23–30.
Zlobin A, Jang M, Miele L . Toward the rational design of cell fate modifiers: notch signaling as a target for novel biopharmaceuticals. Curr Pharm Biotechnol 2000; 1: 83–106.
Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ et al. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res 2002; 8: 2505–2511.
Adams J . Potential for proteasome inhibition in the treatment of cancer. Drug Discov Today 2003; 8: 307–315.
Acknowledgements
This work was supported by Grant No. P15100 from the Austrian Science Foundation, from the Austrian National Bank, ‘Jubilaeumsfonds’-project No. 9448, and from the ‘Fellinger Krebsforschungsfonds’.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
Rights and permissions
About this article
Cite this article
Duechler, M., Shehata, M., Schwarzmeier, J. et al. Induction of apoptosis by proteasome inhibitors in B-CLL cells is associated with downregulation of CD23 and inactivation of Notch2. Leukemia 19, 260–267 (2005). https://doi.org/10.1038/sj.leu.2403592
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403592
Keywords
This article is cited by
-
High-Risk Mantle Cell Lymphoma in the Era of Novel Agents
Current Hematologic Malignancy Reports (2021)
-
(Immuno)proteasomes as therapeutic target in acute leukemia
Cancer and Metastasis Reviews (2017)
-
Combined antitumor effect of γ-secretase inhibitor and ABT-737 in Notch-expressing non-small cell lung cancer
International Journal of Clinical Oncology (2017)
-
Inhibition of Notch and HIF enhances the antitumor effect of radiation in Notch expressing lung cancer
International Journal of Clinical Oncology (2017)
-
Cross talk between EBV and telomerase: the role of TERT and NOTCH2 in the switch of latent/lytic cycle of the virus
Cell Death & Disease (2015)