Abstract
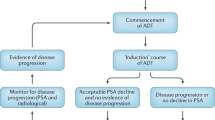
Androgen deprivation therapy (ADT) is first-line treatment for metastatic prostate cancer (PCa). Gonadotrophin-releasing hormone (GnRH) agonists are the most commonly used ADT but have several theoretical physiologic disadvantages (e.g. initial testosterone surge, potential microsurges upon repeat administration). Testosterone surge delays the intended serologic endpoint of testosterone suppression and may exacerbate clinical symptoms. GnRH antagonists were developed with a view toward overcoming these potential adverse physiologic events. This review evaluates GnRH agonists and antagonists, assessing the potential future role of antagonists in PCa and strategies to minimize ADT adverse events (AEs). Evidence was identified via PubMed search (by GnRH agent and other ADT-related terms), from review article bibliographies, and authors’ therapy area knowledge, with articles included by author consensus. Degarelix shows similar efficacy to a GnRH agonist in achieving and maintaining castration, with faster onset and without testosterone surge/microsurges. Phase III data showed that, in the first treatment year, degarelix displayed a lower risk of PSA failure or death (composite endpoint), lower levels of the bone marker serum alkaline phosphatase (in baseline metastatic disease), and fewer musculoskeletal AEs than the agonist leuprolide. Also, crossing over from leuprolide to degarelix after 1 year reduced the risk of PSA failure or death. ADT displays an AE spectrum which can impact quality of life as well as causing significant morbidities. Strategies to improve ADT tolerability have become increasingly important including: a holistic management approach, improved diet and exercise, more specific monitoring to detect and prevent testosterone depletion toxicities, and intermittent ADT allowing hormonal recovery between treatment periods. Clinical studies suggest possible benefits of GnRH antagonists over agonists based on different mechanisms of action. GnRH antagonists should now be considered as an alternative first-line ADT option in advanced PCa. Intermittent ADT and a holistic treatment approach are promising strategies to improve ADT tolerability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD et al. Guidelines on prostate cancer. 2012; Available from: http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf. Accessed April 2012.
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology™. Prostate Cancer. National Comprehensive Cancer Network: Fort Washington, PA, Version 2.2012. Available from: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed April 2012.
Payne H, Mason M . Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: recent developments. Br J Cancer 2011; 105: 1628–1634.
Lee WR . The role of androgen deprivation therapy combined with prostate brachytherapy. Urology 2002; 60 (3 Suppl 1): 39–44.
Zinner NR, Bidair M, Centeno A, Tomera K . Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology 2004; 64: 1177–1181.
Sharifi N, Gulley JL, Dahut WL . An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer 2010; 17: R305–R315.
Damber J-E . Endocrine therapy for prostate cancer. Acta Oncologica 2005; 44: 605–609.
Huhtaniemi I, White R, McArdle CA, Persson BE . Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab 2009; 20: 43–50.
Drudge-Coates L . GnRH blockers: a changing paradigm in the management of prostate cancer. Int J Urological Nursing 2009; 3: 85–92.
Van Poppel H . LHRH agonists versus GnRH antagonists for the treatment of prostate cancer. Belgian J Med Oncol 2010; 4: 18–22.
Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008; 102: 1531–1538.
Van Poppel H, Tombal B, de la Rosette JL, Persson BE, Jensen JK, Kold Olesen T . Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker—results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer. Eur Urol 2008; 54: 805–813.
Belanger A, Brochu M, Cliche J . Levels of plasma steroid glucuronides in intact and castrated men with prostatic cancer. J Clin Endocrinol Metab 1986; 62: 812–815.
Tomic R . Pituitary function after orchiectomy in patients with or without earlier estrogen treatment for prostatic carcinoma. J Endocrinol Invest 1987; 10: 479–482.
Varenhorst E, Wallentin L, Carlström K . The effects of orchidectomy, estrogens, and cyproterone acetate on plasma testosterone, LH, and FSH concentrations in patients with carcinoma of the prostate. Scand J Urol Nephrol 1982; 16: 31–36.
Andò S, Giacchetto C, Canonaco M, Aquila S, Valenti A, Beraldi E et al. Effects of castration on androstenedione, testosterone and dihydrotestosterone plasma levels in adult male rats. Horm Res 1986; 23: 122–127.
Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev 2005; 26: 361–379.
Bhasin S, Berman N, Swerdloff RS . Follicle-stimulating hormone (FSH) escape during chronic gonadotropin-releasing hormone (GnRH) agonist and testosterone treatment. J Androl 1994; 15: 386–391.
Wong SL, Lau DT-W, Baughman SA, Menchaca D, Garnick MB . Pharmacokinetics and pharmacodynamics of abarelix, a gonadotropin-releasing hormone antagonist, after subcutaneous continuous infusion in patients with prostate cancer. Clin Pharmacol Ther 2003; 73: 304–311.
Garnick MB, Campion M . Abarelix Depot, a GnRH antagonist, v LHRH superagonists in prostate cancer: differential effects on follicle-stimulating hormone. Abarelix Depot study group. Mol Urol 2000; 4: 275.
Mahler C . Is disease flare a problem? Cancer 1993; 72: 3799–3802.
Tombal B, Berges R . Optimal control of testosterone: a clinical case-based approach of modern androgen-deprivation therapy. Eur Urol Suppl 2008; 7: 15–21.
Tombal B . Appropriate castration with luteinising hormone releasing hormone (LHRH) agonists: what is the optimal level of testosterone? Eur Urol Suppl 2005; 4: 14–19.
Morote J, Orsola A, Planas J, Trilla E, Raventos CX, Cecchini L et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol 2007; 178: 1290–1295.
Mongiat-Artus P, Teillac P . Abarelix: the first gonadotrophin-releasing hormone antagonist for the treatment of prostate cancer. Expert Opin Pharmacother 2004; 5: 2171–2179.
Perachino M, Cavalli V, Bravi F . Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int 2010; 105: 648–651.
Mahler C, Verhelst J, Chaban M, Denis L . Prolactin and pituitary gonadotropin values and responses to acute luteinizing hormone-releasing hormone (LHRH) challenge in patients having long-term treatment with a depot LHRH analogue. Cancer 1991; 67: 557–559.
Khan MS, O’Brien A . An evaluation of pharmacokinetics and pharmacodynamics of leuprorelin acetate 3M-depot in patients with advanced and metastatic carcinoma of the prostate. Urol Int 1998; 60: 33–40.
Huhtaniemi I, Venho P, Jacobi G, Rannikko S . Response of circulating gonadtropin levels to GnRH agonist treatment in prostatic cancer. J Androl 1991; 12: 46–53.
Ben-Josef E, Yang S Y, Ji TH, Bidart JM, Garde SV, Chopra DP et al. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J Urol 1999; 161: 970–976.
Huhtaniemi I . Are gonadotrophins tumorigenic—a critical review of clinical and experimental data. Mol Cell Endocrinol 2010; 329: 56–61.
Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 2010; 363: 1621–1630.
Mariani S, Salvatori L, Basciani S, Arizzi M, Franco G, Petrangeli E et al. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J Urol 2006; 175: 2072–2077.
Porter AT, Ben-Josef E . Humoral mechanisms in prostate cancer: a role for FSH. Urol Oncol 2001; 6: 131–138.
Canon JG, Kraj B, Sloan G . Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokin 2011; 53: 141–144.
Beer TM, Garzotto M, Eilers KM, Lemmon D, Wersinger EM . Targeting FSH in androgen-independent prostate cancer: abarelix for prostate cancer progressing after orchiectomy. Urology 2004; 63: 342–347.
Beer TM, Garzotto M, Eilers KM, Lemmon D . Phase II study of abarelix depot for androgen independent prostate cancer progression during gonadotropin-releasing hormone agonist therapy. J Urol 2003; 169: 1738–1741.
Beer TM . Experimental use of GnRH antagonists as second-line hormonal therapy. Rev Urol 2004; 6 (Suppl 7): S33–S38.
Debruyne F, Bhat G, Garnick MB . Abarelix for injectable suspension: first-in-class gonadotropin-releasing hormone antagonist for prostate cancer. Future Oncol 2006; 2: 677–696.
Speciality European Pharma. Plenaxis. New drug for prostate cancer. Available from: http://www.specialityeuropeanpharma.com/sepproducts.html. Accessed November 2011.
Broqua P, Riviere PJ, Conn PM, Rivier JE, Aubert ML, Junien JL . Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. J Pharmacol Exp Ther 2002; 301: 95–102.
Koechling W, Hjortkjaer R, Tankó LB . Degarelix, a novel GnRH antagonist, causes minimal histamine release compared with cetrorelix, abarelix and ganirelix in an ex vivo model of human skin samples. Br J Clin Pharmacol 2010; 70: 580–587.
Gittelman M, Pommerville PJ, Persson BE, Jensen JK, Olsen TK . A 1-year, open-label, randomized phase II dose-finding study of degarelix, a novel gonadotropin-releasing hormone (GnRH) receptor blocker, in the treatment of prostate cancer in North America. J Urol 2008; 180: 1986–1992.
Kuhn JM, Billebaud T, Navratil H, Moulonguet A, Fiet J, Grise P et al. Prevention of the transient adverse effects of a gonadotropin-releasing hormone analogue (buserelin) in metastatic prostatic carcinoma by administration of an antiandrogen (nilutamide). N Engl J Med 1989; 321: 413–418.
Anderson J . The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int 2003; 91: 455–461.
Tombal B, Miller K, Boccon-Gibod L, Schrder F, Shore N, Crawford ED et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol 2010; 57: 836–842.
Schröder FH, Tombal B, Miller K, Boccon-Gibod L, Shore ND, Crawford ED et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study. BJU Int 2010; 106: 182–187.
Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N et al. A phase III extension trial with a one-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 2011; 186: 889–897.
Shore N, Moul J, Crawford ED, van der Meulen E, Olesen T, Persson B . Prostate-specific antigen (PSA) progression-free survival (PFS): a comparison of degarelix versus leuprolide in patients with prostate cancer. J Clin Oncol 2011; 29 (suppl 7): 12.
Boccon-Gibod L, van der Meulen E, Persson B-E . An update on the use of gonadotropin-releasing hormone antagonists in prostate cancer. Ther Adv Urol 2011; 3: 127–140.
Oka D, Shiba M, Arai Y, Nakayama M, Takayama H, Inoue H et al. Skin reactions to 3-month depot type of luteinizing hormone-releasing hormone agonist therapy. JMAJ 2006; 49: 48–54.
Crawford ED, Moul JW, Shaw ND, Oleson TK, Persson B-E . Switching from leuprolide to degarelix vs continuous degarelix treatment: effects on long-term prostate-specific antigen control. J Urol 2010; 183 (Suppl): e262, abstract 670.
US Food and Drug Administration (FDA). FDA Drug Safety Communication 10-20-2010. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm. Accessed November 2011.
Levine GN, D’Amico AV, Berger P, Clark PE, Eckel RH, Keating NL et al. Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation 2010; 121: 833–840.
Keating N, O’Malley AJ, Smith MR . Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol 2006; 24: 4448–4456.
Alibhai SM, Duong-Hua M, Sutradhar R, Fleshner NE, Warde P, Cheung AM et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol 2009; 27: 3452–3458.
Smith M, Klotz L, Persson BE, Olesen TK, Wilde AA . Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. J Urol 2010; 184: 2313–2319.
Smith MR, Klotz L, van der Meulen E, Colli E, Tankó L . Gonadotropin-releasing hormone blockers and cardiovascular disease risk: analyses of prospective clinical trials of degarelix. J Urol 2011; 186: 1835–1842.
McLeod D, Zinner N, Tomera K, Gleason D, Fotheringham N, Campion M et al. A phase 3, multicenter, open-label, randomized study of abarelix versus leuprolide acetate in men with prostate cancer. Urology 2001; 58: 756–761.
Trachtenberg J, Gittleman M, Steidle C, Barzell W, Friedel W, Pessis D et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol 2002; 167: 1670–1674.
Selvaggi F, Khoe GSS, Van Cangh P, Jung J-L, Schulman CC, Vallancien G et al. Comparison of abarelix depot (A-D) and goserelin (G) plus bicalutamide (B) in advanced prostate cancer: results of a multicentre, open-label, randomised, phase III study. Eur Urol 2001; 39 (Suppl 5): 78.
Koch M, Steidle C, Brosman S, Centeno A, Gaylis F, Campion M et al. An open-label study of abarelix in men with symptomatic prostate cancer at risk of treatment with LHRH agonists. Urology 2003; 62: 877–882.
Garnick MB, Mottet N . New treatment paradigm for prostate cancer: abarelix initiation therapy for immediate testosterone suppression followed by a luteinizing hormone-releasing hormone agonist. BJU Int 2011. doi:10.1111/j.1464-410X.2011.10708.x.
Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI . Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology 2000; 56: 1021–1024.
Sharma OP, Weinbauer GF, Behre HM, Nieschlag E . The gonadotropin-releasing hormone (GnRH) agonist-induced initial rise of bioactive LH and testosterone can be blunted in a dose-dependent manner by GnRH antagonist in the non-human primate. Urol Res 1992; 20: 317–321.
Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT . Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol 2000; 163: 181–186.
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS . Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005; 352: 154–164.
Oefelein MG, Ricchiuti V, Conrad W, Resnick MI . Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002; 168: 1005–1007.
Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 2002; 87: 599–603.
Smith MR, Lee H, Nathan DM . Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab 2006; 91: 1305–1308.
Shahani S, Braga-Basaria M, Basaria S . Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab 2008; 93: 2042–2049.
Yannucci J, Manola J, Garnick MB, Bhat G, Bubley GJ . The effect of androgen deprivation therapy on fasting serum lipid and glucose parameters. J Urol 2006; 176: 520–525.
Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 2011; 306: 2359–2366.
Northhouse LL, Mood DW, Schafenacker A, Montie JE, Sandler HM, Forman JD et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer 2007; 110: 2809–2818.
Tombal B . A holistic approach to androgen deprivation therapy: treating the cancer without hurting the patient. Urol Int 2009; 83: 373–378.
Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD . Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Australia 2011; 194: 301–306.
Ebeling PR . Clinical practice. Osteoporosis in men. N Engl J Med 2008; 358: 1474–1482.
Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR . Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer 2004; 100: 892–899.
Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009; 361: 745–755.
Saylor PJ, Smith MR . Bone health and prostate cancer. Prostate Cancer Prostatic Dis 2010; 13: 20–27.
Saylor PJ, Smith MR . Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol 2009; 181: 1998–2006.
Tunn U . The current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlight. BJU Int 2007; 99: 19–22.
Gomella LG, Singh J, Lallas C, Trabulsi EJ . Hormone therapy in the management of prostate cancer: evidence-based approaches. Ther Adv Urol 2010; 2: 171–181.
Abrahamsson PA . Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol 2010; 57: 49–59.
Shaw GL, Wilson P, Cuzick J, Prowse DM, Goldenberg SL, Spry NA et al. International study into the use of intermittent hormone therapy in the treatment of carcinoma of the prostate: a meta-analysis of 1446 patients. BJU Int 2007; 99: 1056–1065.
Tunn U . Can intermittent hormone therapy fulfil its promise? Eur Urol Suppl 2008; 7: 752–757.
Calais da Silva FE, Bono AV, Whelan P, Brausi M, Marques Queimadelos A, Martin JA et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol 2009; 55: 1269–1277.
Klotz L, O’Callaghan CJ, Ding K, Dearnaley DP, Higano CS, Horwitz EM et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with PSA progression after radical therapy: NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013. J Clin Oncol 2011; 29 (Suppl 7): 3.
NICE. Prostate Cancer: Diagnosis and Treatment 2008. Available online at: www.nice.org.uk/nicemedia/live/11924/39687/39687.pdf. Accessed November 2011.
Clarke NW, Marberger M . The motion: GnRH antagonists are the new way forward in hormonal therapy. Eur Urol 2010; 57: 534–537.
Williams SG, Duchesne GM, Millar JL, Pratt GR . Both pretreatment prostate-specific antigen level and posttreatment biochemical failure are independent predictors of overall survival after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2004; 60: 1082–1087.
Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulker J, Crawford ED et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol 2006; 24: 3984–3990.
Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009; 27: 2450–2456.
Herbst KL, Coviello AD, Page ST, Amory JK, Anawalt BD, Bremner WJ . Acyline, a gonadotropin releasing-hormone antagonist suppresses gonadotropins and testosterone for 15 days after a single dose. J Clin Endocrinol Metab 2004; 89: 5959–5965.
Amory JK, Leonard TW, Page ST, O’Toole E, McKenna MJ, Bremner WJ . Oral administration of the GnRH antagonist acyline, in a GIPET-enhanced tablet form, acutely suppresses serum testosterone in normal men: single-dose pharmacokinetics and pharmacodynamics. Cancer Chemother Pharmacol 2009; 64: 641–645.
Reuters. Key developments, Ardana plc: Ardana plc announces preliminary phase II repeat dose results for teverelix LA in prostate cancer. 2008. Available at: http://www.reuters.com/article/2008/04/09/idUS59548+09-Apr-2008+RNS20080409. (Accessed December 2011).
Festuccia C, Dondi D, Piccolella M, Locatelli A, Gravina GL, Tombolini V et al. Ozarelix, a fourth generation GnRH antagonist, induces apoptosis in hormone refractory androgen receptor negative prostate cancer cells modulating expression and activity of death receptors. Prostate 2010; 70: 1340–1349.
Gonzalez-Barcena D, Vadillo-Buenfil M, Gomez-Orta F, Fuentes Garcia M, Cardenas-Cornejo I, Graef-Sanchez A et al. Responses to the antagonistic analog of LH-RH (SB-75, cetrorelix) in patients with benign prostatic hyperplasia and prostatic cancer. Prostate 1994; 24: 84–92.
Gonzalex-Barcena D, Cardenas-Cornejo I, Vadillo-Buenfil M, Comaru-Schally AM, Cortex-Moralex A, Schally AV et al. Luteinizing hormone releasing hormone antagonist cetrorelix as primary single therapy in patients with advanced prostatic cancer and paraplegia due to metastatic invasion of spinal cord. Urology 1995; 45: 275–281.
Acknowledgements
The authors thank Marc B Garnick (Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA) for his contribution to the abarelix component of the manuscript. Medical writing assistance (funded by Ferring Pharmaceuticals) was provided by Thomas Lavelle of Bioscript Stirling Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Neal D Shore: Consultant: Ferring, Sanofi, Watson, Astellas, Dendreon, Janssen, Amgen. Per-Anders Abrahamsson: no financial interest or conflicts with regard to the compounds included in this manuscript. John Anderson: previously received honoraria from Ferring Pharmaceuticals. E David Crawford: is an advisor to Ferring Pharmaceuticals, GlaxoSmithKline, Centocor and Sanofi-Aventis, and has been a meeting participant for Aureon. Paul Lange: no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shore, N., Abrahamsson, PA., Anderson, J. et al. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer Prostatic Dis 16, 7–15 (2013). https://doi.org/10.1038/pcan.2012.25
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2012.25