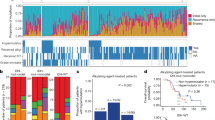
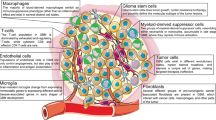
Abstract
In spite of relentless efforts to devise new treatment strategies, primary glioblastomas invariably recur as aggressive, therapy-resistant relapses and patients rapidly succumb to these tumors. Many therapeutic agents are first tested in clinical trials involving recurrent glioblastomas. Remarkably, however, fundamental knowledge on the biology of recurrent glioblastoma is just slowly emerging. Here, we review current knowledge on recurrent glioblastoma and ask whether and how therapies change intra-tumor heterogeneity, molecular traits and growth pattern of glioblastoma, and to which extent this information can be exploited for therapeutic decision-making. We conclude that the ability to characterize and predict therapy-induced changes in recurrent glioblastoma will determine, whether, one day, glioblastoma can be contained in a state of chronic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987–996.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009; 10: 459–466.
Weller M, Cloughesy T, Perry JR, Wick W . Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro-oncology 2013; 15: 4–27.
Marucci G, Fabbri PV, Morandi L, De Biase D, Di Oto E, Tallini G et al. Pathological spectrum in recurrences of glioblastoma multiforme. Pathologica 2015; 107: 1–8.
Kim J, Lee I-H, Cho HJ, Park C-K, Jung Y-S, Kim Y et al. Spatiotemporal Evolution of the Primary Glioblastoma Genome. Cancer Cell 2015; 28: 318–328.
Kim H, Zheng S, Amini SS, Virk SM, Mikkelsen T, Brat DJ et al. Whole-genome and multisector exome sequencing of primary and post-treatment glioblastoma reveals patterns of tumor evolution. Genome Res 2015; 25: 316–327.
Pistollato F, Abbadi S, Rampazzo E, Persano L, Della Puppa A, Frasson C et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells 2010; 28: 851–862.
Glas M, Rath BH, Simon M, Reinartz R, Schramme A, Trageser D et al. Residual tumor cells are unique cellular targets in glioblastoma. Ann Neurol 2010; 68: 264–269.
Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR et al. The somatic genomic landscape of glioblastoma. Cell 2013; 155: 462–477.
Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 2014; 343: 189–193.
Burger PC, Dubois PJ, Schold SC Jr, Smith KR Jr, Odom GL, Crafts DC et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 1983; 58: 159–169.
De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, Chiesa S et al. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg 2013; 115: 37–43.
Van Nifterik KA, Elkhuizen PHM, van Andel RJ, Stalpers LJA, Leenstra S, Lafleur MVM et al. Genetic profiling of a distant second glioblastoma multiforme after radiotherapy: recurrence or second primary tumor? J Neurosurg 2006; 105: 739–744.
Reis RM, Herva R, Brandner S, Koivukangas J, Mironov N, Bär W et al. Second primary glioblastoma. J Neuropathol Exp Neurol 2001; 60: 208–215.
Martinez R, Schackert HK, von Kannen S, Lichter P, Joos S, Schackert G . Independent molecular development of metachronous glioblastomas with extended intervening recurrence-free interval. Brain Pathol 2003; 13: 598–607.
Stark AM, Doukas A, Hugo H-H, Mehdorn HM . The expression of mismatch repair proteins MLH1, MSH2 and MSH6 correlates with the Ki67 proliferation index and survival in patients with recurrent glioblastoma. Neurol Res 2010; 32: 816–820.
Stark AM, Witzel P, Strege RJ, Hugo H-H, Mehdorn HM . p53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J Neurol Neurosurg Psychiatr 2003; 74: 779–783.
Martinez R, Setien F, Voelter C, Casado S, Quesada MP, Schackert G et al. CpG island promoter hypermethylation of the pro-apoptotic gene caspase-8 is a common hallmark of relapsed glioblastoma multiforme. Carcinogenesis 2007; 28: 1264–1268.
Martinez R, Rohde V, Schackert G . Different molecular patterns in glioblastoma multiforme subtypes upon recurrence. J Neurooncol 2010; 96: 321–329.
Bilzer T, Stavrou D, Wechsler W, Wöhler B, Keiditsch E . Antigen variation in a human glioblastoma: from the primary tumor to the second recurrence, permanent cell line and xenotransplantation tumors. Anticancer Res 1991; 11: 547–553.
Shinsato Y, Furukawa T, Yunoue S, Yonezawa H, Minami K, Nishizawa Y et al. Reduction of MLH1 and PMS2 confers temozolomide resistance and is associated with recurrence of glioblastoma. Oncotarget 2013; 4: 2261–2270.
Ilhan-Mutlu A, Wöhrer A, Berghoff AS, Widhalm G, Marosi C, Wagner L et al. Comparison of microRNA expression levels between initial and recurrent glioblastoma specimens. J Neurooncol 2013; 112: 347–354.
Inda M-M, Bonavia R, Mukasa A, Narita Y, Sah DWY, Vandenberg S et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 2010; 24: 1731–1745.
Snuderl M, Fazlollahi L, Le LP, Nitta M, Zhelyazkova BH, Davidson CJ et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell 2011; 20: 810–817.
Szerlip NJ, Pedraza A, Chakravarty D, Azim M, McGuire J, Fang Y et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci USA 2012; 109: 3041–3046.
Sottoriva A, Spiteri I, Piccirillo SGM, Touloumis A, Collins VP, Marioni JC et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 2013; 110: 4009–4014.
Andor N, Harness JV, Müller S, Mewes HW, Petritsch C . EXPANDS: expanding ploidy and allele frequency on nested subpopulations. Bioinformatics 2014; 30: 50–60.
Cancer Genome Atlas Research Network. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T et al. Identification of human brain tumour initiating cells. Nature 2004; 432: 396–401.
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006; 444: 756–760.
Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell 2009; 15: 501–513.
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006; 5: 67.
Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY et al. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol 2009; 94: 1–19.
Lathia JD, Hitomi M, Gallagher J, Gadani SP, Adkins J, Vasanji A et al. Distribution of CD133 reveals glioma stem cells self-renew through symmetric and asymmetric cell divisions. Cell Death Dis 2011; 2: e200.
Richichi C, Brescia P, Alberizzi V, Fornasari L, Pelicci G . Marker-independent method for isolating slow-dividing cancer stem cells in human glioblastoma. Neoplasia 2013; 15: 840–847.
Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H et al. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain 2011; 134: 1331–1343.
Campos B, Gal Z, Baader A, Schneider T, Sliwinski C, Gassel K et al. Aberrant self-renewal and quiescence contribute to the aggressiveness of glioblastoma. J Pathol 2014; 234: 23–33.
Chen J, Li Y, Yu T-S, McKay RM, Burns DK, Kernie SG et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 2012; 488: 522–526.
Campos B, Herold-Mende CC . Insight into the complex regulation of CD133 in glioma. Int J Cancer 2011; 128: 501–510.
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 2008; 14: 123–129.
Tamura K, Aoyagi M, Ando N, Ogishima T, Wakimoto H, Yamamoto M et al. Expansion of CD133-positive glioma cells in recurrent de novo glioblastomas after radiotherapy and chemotherapy. J Neurosurg 2013; 119: 1145–1155.
Pallini R, Ricci-Vitiani L, Montano N, Mollinari C, Biffoni M, Cenci T et al. Expression of the stem cell marker CD133 in recurrent glioblastoma and its value for prognosis. Cancer 2011; 117: 162–174.
Qazi MA, Vora P, Venugopal C, McFarlane N, Subapanditha MK, Murty NK et al. A novel stem cell culture model of recurrent glioblastoma. J Neurooncol 2015, e-pub ahead of print 23 October 2015 10.1007/s11060-015-1951-6.
Smets T, Lawson TM, Grandin C, Jankovski A, Raftopoulos C . Immediate post-operative MRI suggestive of the site and timing of glioblastoma recurrence after gross total resection: a retrospective longitudinal preliminary study. Eur Radiol 2013; 23: 1467–1477.
Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010; 17: 510–522.
Christmann M, Nagel G, Horn S, Krahn U, Wiewrodt D, Sommer C et al. MGMT activity, promoter methylation and immunohistochemistry of pretreatment and recurrent malignant gliomas: a comparative study on astrocytoma and glioblastoma. Int J Cancer 2010; 127: 2106–2118.
Brandes AA, Tosoni A, Franceschi E, Sotti G, Frezza G, Amistà P et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol 2009; 27: 1275–1279.
Iwamoto FM, Abrey LE, Beal K, Gutin PH, Rosenblum MK, Reuter VE et al. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology 2009; 73: 1200–1206.
Poulsen HS, Urup T, Michaelsen SR, Staberg M, Villingshøj M, Lassen U . The impact of bevacizumab treatment on survival and quality of life in newly diagnosed glioblastoma patients. Cancer Manag Res 2014; 6: 373–387.
Furuta T, Nakada M, Misaki K, Sato Y, Hayashi Y, Nakanuma Y et al. Molecular analysis of a recurrent glioblastoma treated with bevacizumab. Brain Tumor Pathol 2013; 31: 32–39.
Mittal D, Gubin MM, Schreiber RD, Smyth MJ . New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol 2014; 27: 16–25.
Sampson JH, Heimberger AB, Archer GE, Aldape KD, Friedman AH, Friedman HS et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 2010; 28: 4722–4729.
Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SAA, Fack F et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc Natl Acad Sci USA 2011; 108: 3749–3754.
Sathornsumetee S, Cao Y, Marcello JE, Herndon JE, McLendon RE, Desjardins A et al. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J Clin Oncol 2008; 26: 271–278.
Yao X, Ping Y, Liu Y, Chen K, Yoshimura T, Liu M et al. Vascular endothelial growth factor receptor 2 (VEGFR-2) plays a key role in vasculogenic mimicry formation, neovascularization and tumor initiation by Glioma stem-like cells. PLoS ONE 2013; 8: e57188.
Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y et al. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-oncology 2013; 15: 1079–1087.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
Literature review and conception, drafting the article and critical revision, and final approval of the version to be published: BC, LRO, TU, HSP.
Rights and permissions
About this article
Cite this article
Campos, B., Olsen, L., Urup, T. et al. A comprehensive profile of recurrent glioblastoma. Oncogene 35, 5819–5825 (2016). https://doi.org/10.1038/onc.2016.85
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.85
This article is cited by
-
Superenhancers as master gene regulators and novel therapeutic targets in brain tumors
Experimental & Molecular Medicine (2023)
-
Exosome-based nanoimmunotherapy targeting TAMs, a promising strategy for glioma
Cell Death & Disease (2023)
-
Spatial proteomics of tumor microenvironments reveal why location matters
Nature Immunology (2023)
-
The role of chromatin remodeler SMARCA4/BRG1 in brain cancers: a potential therapeutic target
Oncogene (2023)
-
Circular RNA circ_0000741/miR-379-5p/TRIM14 signaling axis promotes HDAC inhibitor (SAHA) tolerance in glioblastoma
Metabolic Brain Disease (2023)