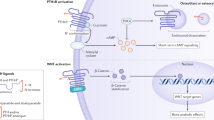
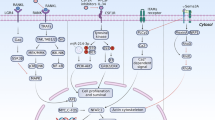
Abstract
Postmenopausal osteoporosis is a disease of high bone remodeling, with an imbalance of bone resorption over bone formation, resulting in decreased bone mineral density and disruption of bone microarchitecture. With our improved understanding of the molecular and cellular regulators and mediators of bone remodeling, new targets for therapeutic intervention have been identified. Receptor activator of nuclear factor κB ligand (RANKL) is the principal regulator of osteoclast differentiation, activity, and survival; denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and is approved for the treatment of women with postmenopausal osteoporosis at high risk of fractures. Cathepsin K is a protease produced by activated osteoclasts that degrades the protein matrix of bone. An inhibitor of cathepsin K, odanacatib, is in phase III clinical trials for the treatment of postmenopausal osteoporosis; it decreases bone resorption while seeming to suppress bone formation less than other antiresorptive agents. Sclerostin is a cytokine produced by osteocytes that inhibits osteoblastic bone formation; investigational monoclonal antibodies to sclerostin, such as AMG 785, have osteoanabolic properties with the potential to improve clinical outcomes in patients with osteoporosis. These and other novel interventions that target newly recognized regulators of bone remodeling are promising agents for the treatment of osteoporosis.
Key Points
-
Postmenopausal osteoporosis is a disease of excessive bone remodeling, with an imbalance of bone resorption greater than bone formation
-
Understanding of the regulation of bone remodeling has led to the identification of novel pharmacological targets for the treatment of postmenopausal osteoporosis
-
Denosumab is a fully human monoclonal antibody against receptor activator of nuclear factor κB ligand (RANKL) that is an approved treatment for women with postmenopausal osteoporosis at high risk of fractures
-
Odanacatib, an investigational inhibitor of cathepsin K, increases bone mineral density (BMD) with a mode of action that might partially uncouple bone resorption and formation
-
Investigational antibodies against sclerostin, such as AMG 785, increase BMD by stimulating bone formation and reducing bone resorption
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klibanski, A. et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–795 (2001).
Kanis, J. A., on behalf of the World Health Organization Scientific Group. Assessment of Osteoporosis at the Primary Health-care Level (World Health Organization Collaborating Centre for Metabolic Bone Diseases, University of Sheffield, UK, 2008).
National Osteoporosis Foundation. America's Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation (National Osteoporosis Foundation, Washington, DC, 2002).
US Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General (US Department of Health and Human Services, Office of the Surgeon General, Rockville, MD, 2004).
Riggs, B. L. & Melton, L. J. III. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone 17 (Suppl.), 505S–511S (1995).
Cooper, C., Atkinson, E. J., Jacobsen, S. J., O'Fallon, W. M. & Melton, L. J. III. Population-based study of survival after osteoporotic fractures. Am. J. Epidemiol. 137, 1001–1005 (1993).
Burge, R. et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res. 22, 465–475 (2007).
Kanis, J. A. & Johnell, O. Requirements for DXA for the management of osteoporosis in Europe. Osteoporos. Int. 16, 229–238 (2005).
Felsenberg, D. & Boonen, S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin. Ther. 27, 1–11 (2005).
Lewiecki, E. M. Update on bone density testing. Curr. Osteoporos. Rep. 3, 136–142 (2005).
World Health Organization. FRAX WHO Fracture Risk Assessment Tool [online].
MacLean, C. et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann. Intern. Med. 148, 197–213 (2008).
McClung, M. R. et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch. Intern. Med. 165, 1762–1768 (2005).
Meunier, P. J. et al. The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N. Engl. J. Med. 350, 459–468 (2004).
Delmas, P. D. et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J. Bone Miner. Res. 20, 557–563 (2005).
Panneman, M. J., Lips, P., Sen, S. S. & Herings, R. M. Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos. Int. 15, 120–124 (2004).
Cramer, J. A., Gold, D. T., Silverman, S. L. & Lewiecki, E. M. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos. Int. 18, 1023–1031 (2007).
Lewiecki, E. M. Review of guidelines for bone mineral density testing and treatment of osteoporosis. Curr. Osteoporos. Rep. 3, 75–83 (2005).
Theoleyre, S. et al. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 15, 457–475 (2004).
Zhao, H. et al. Critical role of β3 integrin in experimental postmenopausal osteoporosis. J. Bone Miner. Res. 20, 2116–2123 (2005).
McHugh, K. P. et al. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J. Clin. Invest. 105, 433–440 (2000).
Yadav, V. K. et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135, 825–837 (2008).
Oury, F. et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 24, 2330–2342 (2010).
Bonewald, L. F. in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism (ed Rosen, C. J.) 7th edn Ch. 4 22–27 (American Society for Bone and Mineral Research, Washington, DC, 2008).
Lewiecki, E. M. RANK ligand inhibition with denosumab for the management of osteoporosis. Expert Opin. Biol. Ther. 6, 1041–1050 (2006).
Cummings, S. R. et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 361, 756–765 (2009).
Rodan, S. B. & Duong, L. T. Cathepsin K—a new molecular target for osteoporosis. IBMS BoneKEy 5, 16–24 (2008).
Kim, T. S. & Tasker, A. S. Non-covalent cathepsin K inhibitors for the treatment of osteoporosis. Curr. Top. Med. Chem. 6, 355–360 (2006).
US National Library of Medicine. ClinicalTrials.gov [online], (2007).
Eastell, R. et al. Safety and efficacy of the cathepsin K inhibitor ONO-5334 in postmenopausal osteoporosis: the OCEAN study. J. Bone Miner. Res. 26, 1303–1312 (2011).
Velcura Therapeutics. Pipeline [online] (2011).
Medivir. Press Conference: Medivir sharpens the strategic focus and strengthens its financial position [online] (2009).
Bone, H. G. et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: a two-year study in postmenopausal women with low bone density. J. Bone Miner. Res. 25, 937–947 (2010).
McClung, M. et al. A randomized, double-blind, placebo-controlled study of odanacatib (MK-822) in the treatment of postmenopausal women with low bone mineral density: 24 month results. J. Bone Miner. Res. 23, S82 (2008).
Lewiecki, E. M. Odanacatib, a cathepsin K inhibitor for the treatment of osteoporosis and other skeletal disorders associated with excessive bone remodeling. IDrugs 12, 799–809 (2009).
Veverka, V. et al. Characterization of the structural features and interactions of sclerostin: molecular insight into a key regulator of Wnt-mediated bone formation. J. Biol. Chem. 284, 10890–10900 (2009).
Babcook, J. S., Leslie, K. B., Olsen, O. A., Salmon, R. A. & Schrader, J. W. A novel strategy for generating monoclonal antibodies from single, isolated lymphocytes producing antibodies of defined specificities. Proc. Natl Acad. Sci. USA 93, 7843–7848 (1996).
Li, X. et al. Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J. Bone Miner. Res. 24, 578–588 (2009).
Ominsky, M. S. et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J. Bone Miner. Res. 25, 948–959 (2010).
Rey, J. P. & Ellies, D. L. Wnt modulators in the biotech pipeline. Dev. Dyn. 239, 102–114 (2010).
Padhi, D., Jang, G., Stouch, B., Fang, L. & Posvar, E. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J. Bone Miner. Res. 26, 19–26 (2011).
Amgen. Amgen and UCB Announce Positive Phase 2 Results of AMG 785/CDP7851 in Patients With Postmenopausal Osteoporosis (PMO) [online] (2011).
Yadav, V. K. et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med. 16, 308–312 (2010).
Frost, M. et al. Patients with high-bone-mass phenotype owing to Lrp5–T253I mutation have low plasma levels of serotonin. J. Bone Miner. Res. 25, 673–675 (2010).
Wimalawansa, S. J. Nitric oxide: novel therapy for osteoporosis. Expert Opin. Pharmacother. 9, 3025–3044 (2008).
Jamal, S. A., Browner, W. S., Bauer, D. C. & Cummings, S. R. Intermittent use of nitrates increases bone mineral density: the study of osteoporotic fractures. J. Bone Miner. Res. 13, 1755–1759 (1998).
Jamal, S. A., Cummings, S. R. & Hawker, G. A. Isosorbide mononitrate increases bone formation and decreases bone resorption in postmenopausal women: a randomized trial. J. Bone Miner. Res. 19, 1512–1517 (2004).
Rejnmark, L., Vestergaard, P. & Mosekilde, L. Decreased fracture risk in users of organic nitrates: a nationwide case–control study. J. Bone Miner. Res. 21, 1811–1817 (2006).
Jamal, S. A., Hamilton, C. J., Eastell, R. & Cummings, S. R. Effect of nitroglycerin ointment on bone density and strength in postmenopausal women: a randomized trial. JAMA 305, 800–807 (2011).
Wimalawansa, S. J., Grimes, J. P., Wilson, A. C. & Hoover, D. R. Transdermal nitroglycerin therapy may not prevent early postmenopausal bone loss. J. Clin. Endocrinol. Metab. 94, 3356–3364 (2009).
Glantschnig, H. et al. A rate-limiting role for DKK1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J. Pharmacol. Exp. Ther. 338, 568–578 (2011).
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Heath, D. J. et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J. Bone Miner. Res. 24, 425–436 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2008).
Widler, L. Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med. Chem. 3, 535–547 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2008).
[No authors listed] In brief: delayed-release risedronate (Atelvia). Med. Lett. Drugs Ther. 53, 24 (2011).
Karsdal, M. A. et al. Lessons learned from the development of oral calcitonin: the first tablet formulation of a protein in phase III clinical trials. J. Clin. Pharmacol. 51, 460–471 (2011).
Cosman, F. et al. Effect of transdermal teriparatide administration on bone mineral density in postmenopausal women. J. Clin. Endocrinol. Metab. 95, 151–158 (2010).
John, M. R. et al. A novel oral parathyroid hormone formulation, PTH134, demonstrated a potential therapeutically relevant pharmacokinetic and safety profile compared with teriparatide s.c. in healthy postmenopausal women after a single dose [abstract]. Arthritis Rheum. 60, 887 (2009).
Shoyele, S. A., Sivadas, N. & Cryan, S. A. The effects of excipients and particle engineering on the biophysical stability and aerosol performance of parathyroid hormone (1–34) prepared as a dry powder for inhalation. AAPS PharmSciTech 12, 304–311 (2011).
Lindsay, R. Preventing osteoporosis with a tissue selective estrogen complex (TSEC) containing bazedoxifene/conjugated estrogens (BZA/CE). Osteoporos. Int. 22, 447–451 (2011).
Pinkerton, J. V. & Stovall, D. W. Bazedoxifene when paired with conjugated estrogens is a new paradigm for treatment of postmenopausal women. Expert Opin. Investig. Drugs 19, 1613–1621 (2010).
Black, D. M. et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N. Engl. J. Med. 353, 555–565 (2005).
Kurland, E. S. et al. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1–34)]. Osteoporos. Int. 15, 992–997 (2004).
Ettinger, B., San, M. J., Crans, G. & Pavo, I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J. Bone Miner. Res. 19, 745–751 (2004).
Cosman, F. et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1–34)] in postmenopausal osteoporosis. J. Bone Miner. Res. 26, 503–511 (2011).
Cosman, F. et al. Daily and cyclic parathyroid hormone in women receiving alendronate. N. Engl. J. Med. 353, 566–575 (2005).
Leder, B. Z. et al. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J. Clin. Endocrinol. Metab. 94, 2915–2921 (2009).
Black, D. M. et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348, 1535–1541 (1996).
Harris, S. T. et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. JAMA 282, 1344–1352 (1999).
Chesnut, C. H. III et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J. Bone Miner. Res. 19, 1241–1249 (2004).
Black, D. M. et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 356, 1809–1822 (2007).
Ettinger, B. et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene—results from a 3-year randomized clinical trial. JAMA 282, 637–645 (1999).
Chesnut, C. H. III et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am. J. Med. 109, 267–276 (2000).
Neer, R. M. et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).
Greenspan, S. L. et al. Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann. Intern. Med. 146, 326–339 (2007).
US National Library of Medicine. ClinicalTrials.gov [online], (2009).
Fukumoto, S. Antagonist for calcium-sensing receptor. JTT-305/MK-5442 [Japanese]. Clin. Calcium 21, 89–93 (2011).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
E. M. Lewiecki has received grant/research support from Amgen, Eli Lilly, Novartis, Merck, Warner Chilcott, and Genentech, and served on the scientific advisory board and/or received speakers' bureau from Amgen, Eli Lilly Novartis, Genentech and Merck.
Rights and permissions
About this article
Cite this article
Lewiecki, E. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat Rev Rheumatol 7, 631–638 (2011). https://doi.org/10.1038/nrrheum.2011.130
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrrheum.2011.130
This article is cited by
-
Long non-coding RNA Homeobox D gene cluster antisense growth-associated long noncoding RNA/microRNA-182-5p/Homeobox protein A10 alleviates postmenopausal osteoporosis via accelerating osteoblast differentiation of bone marrow mesenchymal stem cells
Journal of Orthopaedic Surgery and Research (2023)
-
Nrf2 signaling activation by a small molecule activator compound 16 inhibits hydrogen peroxide-induced oxidative injury and death in osteoblasts
Cell Death Discovery (2022)
-
Activation of cannabinoid receptor type 2-induced osteogenic differentiation involves autophagy induction and p62-mediated Nrf2 deactivation
Cell Communication and Signaling (2020)
-
IL-20 bone diseases involvement and therapeutic target potential
Journal of Biomedical Science (2018)
-
Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis
Nature Communications (2018)