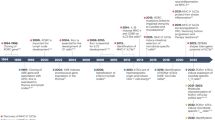
Key Points
-
Dendritic cells (DCs) are heterogeneous. The migratory DCs traffic from tissues to lymph nodes, where they mature. The resident DCs spend their entire lifespan in the lymphoid organs in an immature state until activated by signals reaching these organs. Both migratory and resident DCs can be subdivided into several subtypes with different roles in antigen presentation.
-
Endogenous antigenic peptides (that is, those derived from proteins synthesized by the DCs themselves) are presented on MHC class I and II molecules by all DCs and across all maturational states.
-
Variability in antigen capture (through differential expression of endocytic mechanisms or receptors) can dictate the part played by each DC subset in the presentation of exogenous antigens. Additional differences in the expression of components of the cross-presentation machinery, and in antigen handling, further determine the role of each DC in MHC class II presentation and MHC class I cross-presentation.
-
Resident DCs have important roles in the presentation of antigens from blood pathogens; CD8+ DCs are biased towards MHC class I cross-presentation and CD8− DCs are biased towards MHC class II presentation.
-
Self and pathogen antigens located in peripheral tissues are brought to lymph nodes by migratory DCs, which present the antigens on MHC class II molecules. These antigens can be transferred to resident CD8+ DCs, which then present the antigens on MHC class II molecules, and also cross-present them through the MHC class I pathway.
-
Cooperation between migratory and resident DC subsets allows the exploitation of the intrinsic properties of each DC subset in order to optimize the capacity of the DC network to respond efficiently to different scenarios of infection.
Abstract
Dendritic cells (DCs) comprise several subsets, and their roles in the presentation of antigens derived from pathogens, vaccines and self tissues are now beginning to be elucidated. Differences in location, life cycle and intrinsic abilities to capture, process and present antigens on their MHC class I and class II molecules enable each DC subset to have distinct roles in immunity to infection and in the maintenance of self tolerance. Unexpected interactions among DC subsets have also been revealed. These interactions, which allow the integration of the intrinsic abilities of different DC types, enhance the ability of the DC network to respond to multiple scenarios of infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wilson, N. S. & Villadangos, J. A. Lymphoid organ dendritic cells: beyond the Langerhans cells paradigm. Immunol. Cell Biol. 82, 91–98 (2004).
Steinman, R. M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 9, 271–296 (1991).
Wilson, N. S. & Villadangos, J. A. Regulation of antigen presentation and cross-presentation in the dendritic cell network: facts, hypothesis, and immunological implications. Adv. Immunol. 86, 241–305 (2005).
Iwasaki, A. & Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nature Immunol. 5, 987–995 (2004).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nature Rev. Immunol. 6, 823–835 (2006).
Meylan, E., Tschopp, J. & Karin, M. Intracellular pattern recognition receptors in the host response. Nature 442, 39–44 (2006).
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and Langerhans cells. Nature Rev. Immunol. 2, 77–84 (2002).
Leibundgut-Landmann, S. et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nature Immunol. 8, 630–638 (2007).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Marsland, B. J. et al. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 22, 493–505 (2005).
Albert, M. L., Jegathesan, M. & Darnell, R. B. Dendritic cell maturation is required for the cross-tolerization of CD8+ T cells. Nature Immunol. 2, 1010–1017 (2001).
Sporri, R. & Reis e Sousa, C. Inflammatory mediators are insufficient for full dendritic cell activation and promote expansion of CD4+ T cell populations lacking helper function. Nature Immunol. 6, 163–170 (2005).
Villadangos, J. A. & Heath, W. R. Life cycle, migration and antigen presenting functions of spleen and lymph node dendritic cells: limitations of the Langerhans cells paradigm. Semin. Immunol. 17, 262–272 (2005).
Reis e Sousa, C. Dendritic cells in a mature age. Nature Rev. Immunol. 6, 476–483 (2006).
Shortman, K. & Naik, S. H. Steady-state and inflammatory dendritic-cell development. Nature Rev. Immunol. 7, 19–30 (2007).
Colonna, M., Krug, A. & Cella, M. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14, 373–379 (2002).
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nature Immunol. 5, 1219–1226 (2004).
Liu, Y. J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 23, 275–306 (2005).
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature Rev. Immunol. 5, 617–628 (2005).
Johansson, C. & Kelsall, B. L. Phenotype and function of intestinal dendritic cells. Semin. Immunol. 17, 284–294 (2005).
Walton, K. L., He, J., Kelsall, B. L., Sartor, R. B. & Fisher, N. C. Dendritic cells in germ-free and specific pathogen-free mice have similar phenotypes and in vitro antigen presenting function. Immunol. Lett. 102, 16–24 (2006).
Huang, F. P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–444 (2000). This article shows that migratory DCs constitutively transport apoptotic-cell fragments to the lymph nodes, most likely for the induction of peripheral tolerance in the steady state.
Turnbull, E. & MacPherson, G. Immunobiology of dendritic cells in the rat. Immunol. Rev. 184, 58–68 (2001).
Cavanagh, L. L. et al. Activation of bone marrow-resident memory T cells by circulating, antigen-bearing dendritic cells. Nature Immunol. 6, 1029–1037 (2005).
Bonasio, R. et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nature Immunol. 7, 1092–1100 (2006).
Kabashima, K. et al. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity 22, 439–450 (2005).
Naik, S. H. et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nature Immunol. 7, 663–671 (2006). This article describes the intrasplenic precursor of the lymphoid-organ-resident DC populations and establishes that this DC subset is developmentally distinct from monocyte-derived DCs.
Liu, K. et al. Origin of dendritic cells in peripheral lymphoid organs of mice. Nature Immunol. 8, 578–583 (2007).
Wilson, N. S. et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102, 2187–2194 (2003). In this study, the authors used standard tests of DC maturity to establish that resident and migratory DCs are immature and mature, respectively. This confirmed a clear dichotomy in the life cycle of these two major DC groups.
Henri, S. et al. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167, 741–748 (2001).
Voisine, C., Hubert, F. X., Trinite, B., Heslan, M. & Josien, R. Two phenotypically distinct subsets of spleen dendritic cells in rats exhibit different cytokine production and T cell stimulatory activity. J. Immunol. 169, 2284–2291 (2002).
Zhuang, Y. et al. Characterization of a phenotypically unique population of CD13+ dendritic cells resident in the spleen. Clin. Vaccine Immunol. 13, 1064–1069 (2006).
Jamin, A., Gorin, S., Le Potier, M. F. & Kuntz-Simon, G. Characterization of conventional and plasmacytoid dendritic cells in swine secondary lymphoid organs and blood. Vet. Immunol. Immunopathol. 114, 224–237 (2006).
McIlroy, D. et al. Investigation of human spleen dendritic cell phenotype and distribution reveals evidence of in vivo activation in a subset of organ donors. Blood 97, 3470–3477 (2001). An important study of human splenic DCs that reaches similar conclusions to those in reference 29, which are based on mouse studies.
Inaba, K. et al. The tissue distribution of the B7–2 costimulator in mice: abundant expression on dendritic cells in situ and during maturation in vitro. J. Exp. Med. 180, 1849–1860 (1994).
De Smedt, T. et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184, 1413–1424 (1996).
Reis e Sousa, C. et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J. Exp. Med. 186, 1819–1829 (1997).
Wilson, N. S. et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nature Immunol. 7, 165–172 (2006). This article indicates that T-cell priming against viral infections requires cross-presentation by resident DCs. It also shows that systemic activation of this DC subset impairs subsequent cross-presentation, providing one plausible explanation for the immunosuppression that accompanies sepsis and malaria infection.
Sponaas, A. M. et al. Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 203, 1427–1433 (2006).
Tacke, F. & Randolph, G. J. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 211, 609–618 (2006).
Randolph, G. J., Inaba, K., Robbiani, D. F., Steinman, R. M. & Muller, W. A. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11, 753–761 (1999).
Le Borgne, M. et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24, 191–201 (2006).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nature Immunol. 7, 265–273 (2006).
Leon, B., Lopez-Bravo, M. & Ardavin, C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26, 519–531 (2007). References 42 and 44 describe two clear examples in which newly generated monocyte-derived DCs take over the antigen-presenting functions normally attributed to the pre-existing resident and migratory DC subsets. In reference 42, the monocyte-derived DCs cross-present a model antigen on MHC class I, whereas in reference 44 they present Leishmania spp. antigens on MHC class II.
Krutzik, S. R. et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nature Med. 11, 653–660 (2005).
Serbina, N. V., Salazar-Mather, T. P., Biron, C. A., Kuziel, W. A. & Pamer, E. G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 19, 59–70 (2003).
Yewdell, J. W. & Nicchitta, C. V. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 27, 368–373 (2006).
Strawbridge, A. B. & Blum, J. S. Autophagy in MHC class II antigen processing. Curr. Opin. Immunol. 19, 87–92 (2007).
Veeraswamy, R. K., Cella, M., Colonna, M. & Unanue, E. R. Dendritic cells process and present antigens across a range of maturation states. J. Immunol. 170, 5367–5372 (2003).
Wilson, N. S., El-Sukkari, D. & Villadangos, J. A. Dendritic cells constitutively present self antigens in their immature state in vivo, and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood 103, 2187–2195 (2004).
Steptoe, R. J. et al. Cognate CD4+ help elicited by resting dendritic cells does not impair the induction of peripheral tolerance in CD8+ T cells. J. Immunol. 178, 2094–2103 (2007).
Norbury, C. C., Malide, D., Gibbs, J. S., Bennink, J. R. & Yewdell, J. W. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nature Immunol. 3, 265–271 (2002).
Paludan, C. et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307, 593–596 (2005).
He, Y., Zhang, J., Donahue, C. & Falo, L. D. Jr . Skin-derived dendritic cells induce potent CD8+ T cell immunity in recombinant lentivector-mediated genetic immunization. Immunity 24, 643–656 (2006). In this study, the authors compare the role of migratory and resident DC types in the presentation of antigens that are expressed by a non-cytopathic virus versus a cytopathic one. Infected migratory DCs presenting endogenous antigens were the main inducers of immune responses to the non-cytopathic virus, but resident cross-presenting DCs were the main players in cytopathic viral infection.
Tortorella, D., Gewurz, B. E., Furman, M. H., Schust, D. J. & Ploegh, H. L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000).
Jung, S. et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17, 211–220 (2002).
Heath, W. R. et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004).
Rock, K. L. & Shen, L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol. Rev. 207, 166–183 (2005).
den Haan, J. M., Lehar, S. M. & Bevan, M. J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000). The first demonstration of the unique role of CD8+ DCs in cross-presentation, from the same laboratory that first discovered this phenomenon.
Schulz, O. & Reis e Sousa, C. Cross-presentation of cell-associated antigens by CD8α+ dendritic cells is attributable to their ability to internalize dead cells. Immunology 107, 183–189 (2002).
Iyoda, T. et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195, 1289–1302 (2002).
Valdez, Y. et al. Major histocompatibility complex class II presentation of cell-associated antigen is mediated by CD8α+ dendritic cells in vivo. J. Exp. Med. 195, 683–694 (2002).
Kerksiek, K. M., Niedergang, F., Chavrier, P., Busch, D. H. & Brocker, T. Selective Rac1 inhibition in dendritic cells diminishes apoptotic cell uptake and cross-presentation in vivo. Blood 105, 742–749 (2005).
Schnorrer, P. et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc. Natl Acad. Sci. USA 103, 10729–10734 (2006). A comparison of uptake, MHC class II presentation and MHC class I cross-presentation of different forms of antigen. It concludes that cross-presentation requires a specialized mechanism to handle captured antigens, and that such a mechanism occurs mainly in the CD8+ DC subset.
Yrlid, U. & Wick, M. J. Antigen presentation capacity and cytokine production by murine splenic dendritic cell subsets upon Salmonella encounter. J. Immunol. 169, 108–116 (2002).
Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Caminschi, I. et al. Molecular cloning of a C-type lectin superfamily protein differentially expressed by CD8α− splenic dendritic cells. Mol. Immunol. 38, 365–373 (2001).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Carter, R. W., Thompson, C., Reid, D. M., Wong, S. Y. & Tough, D. F. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J. Immunol. 177, 2276–2284 (2006). In references 68 and 69, the authors elegantly show that CD8− DCs can only present antigens via MHC class II, whereas CD8+ DCs can also deliver antigens to the cross-presentation pathway.
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P. A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007). In this article, the authors conclude that not all forms of endocytosis deliver antigens to the cross-presentation pathway. They show that antigens captured by the mannose receptor, but not those internalized by pinocytosis, are efficiently delivered to putative compartments for cross-presentation.
McKenzie, E. J. et al. Mannose receptor expression and function define a new population of murine dendritic cells. J. Immunol. 178, 4975–4983 (2007).
Edwards, A. D. et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8α+ DC correlates with unresponsiveness to imidazoquinolines. Eur. J. Immunol. 33, 827–833 (2003).
Naik, S. H. et al. Cutting edge: generation of splenic CD8+ and CD8− dendritic cell equivalents in FMS-like tyrosine kinase 3 ligand bone marrow cultures. J. Immunol. 174, 6592–6597 (2005).
Yarovinsky, F. et al. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308, 1626–1629 (2005).
Yarovinsky, F., Kanzler, H., Hieny, S., Coffman, R. L. & Sher, A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity 25, 655–664 (2006). Together with reference 74, this study provides a clear example of the differential involvement of DC subtypes in immune responses to a pathogen that is due to differential expression of one particular receptor. The authors show that only CD8+ DCs express TLR11, which recognizes T. gondii profilin, so only this subset can present the antigen via MHC class II to induce a CD4+ T-cell response against the parasite.
Norbury, C. C. Drinking a lot is good for dendritic cells. Immunology 117, 443–451 (2006).
Manickasingham, S. & Reis e Sousa, C. Microbial and T cell-derived stimuli regulate antigen presentation by dendritic cells in vivo. J. Immunol. 165, 5027–5034 (2000).
Pooley, J. L., Heath, W. R. & Shortman, K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8− dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J. Immunol. 166, 5327–5330 (2001).
Stoitzner, P. et al. Langerhans cells cross-present antigen derived from skin. Proc. Natl Acad. Sci. USA 103, 7783–7788 (2006).
Corbett, A. J. et al. Antigen delivery via two molecules on the CD8− dendritic cell subset induces humoral immunity in the absence of conventional 'danger'. Eur. J. Immunol. 35, 2815–2825 (2005).
El-Sukkari, D. et al. The protease inhibitor cystatin C is differentially expressed among dendritic cell populations, but does not control antigen presentation. J. Immunol. 171, 5003–5011 (2003).
Maehr, R. et al. Asparagine endopeptidase is not essential for class II MHC antigen presentation but is required for processing of cathepsin L in mice. J. Immunol. 174, 7066–7074 (2005).
Chen, X., Reed-Loisel, L. M., Karlsson, L. & Jensen, P. E. H2-O expression in primary dendritic cells. J. Immunol. 176, 3548–3556 (2006).
Fallas, J. L., Yi, W., Draghi, N. A., O' Rourke H, M. & Denzin, L. K. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J. Immunol. 178, 1488–1497 (2007).
Maric, M. et al. Defective antigen processing in GILT-free mice. Science 294, 1361–1365 (2001).
Sinnathamby, G., Maric, M., Cresswell, P. & Eisenlohr, L. C. Differential requirements for endosomal reduction in the presentation of two H2-E(d)-restricted epitopes from influenza hemagglutinin. J. Immunol. 172, 6607–6614 (2004).
Villadangos, J. A., Schnorrer, P. & Wilson, N. S. Control of MHC class II antigen presentation in dendritic cells: a balance between creative and destructive forces. Immunol. Rev. 207, 191–205 (2005).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Lutz, M. B. et al. Intracellular routes and selective retention of antigens in mildly acidic cathepsin D/lysosome-associated membrane protein-1/MHC class II- positive vesicles in immature dendritic cells. J. Immunol. 159, 3707–3716 (1997).
Bergtold, A., Desai, D. D., Gavhane, A. & Clynes, R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 23, 503–514 (2005).
Lennon-Dumenil, A. M. et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J. Exp. Med. 196, 529–540 (2002).
Reich, M. et al. Endocytosis targets exogenous material selectively to cathepsin S in live human dendritic cells, while cell-penetrating peptides mediate nonselective transport to cysteine cathepsins. J. Leukoc. Biol. 81, 990–1001 (2007).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Pu, Z., Lovitch, S. B., Bikoff, E. K. & Unanue, E. R. T cells distinguish MHC-peptide complexes formed in separate vesicles and edited by H2-DM. Immunity 20, 467–476 (2004).
Lovitch, S. B., Esparza, T. J., Schweitzer, G., Herzog, J. & Unanue, E. R. Activation of type B T cells after protein immunization reveals novel pathways of in vivo presentation of peptides. J. Immunol. 178, 122–133 (2007).
Cervi, L., MacDonald, A. S., Kane, C., Dzierszinski, F. & Pearce, E. J. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 172, 2016–2020 (2004).
Engering, A. J. et al. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 27, 2417–2425 (1997).
Tan, M. C. et al. Mannose receptor-mediated uptake of antigens strongly enhances HLA class II-restricted antigen presentation by cultured dendritic cells. Eur. J. Immunol. 27, 2426–2435 (1997).
Shen, L., Sigal, L. J., Boes, M. & Rock, K. L. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21, 155–165 (2004).
Norbury, C. C., Hewlett, L. J., Prescott, A. R., Shastri, N. & Watts, C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3, 783–791 (1995).
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nature Cell Biol. 1, 362–368 (1999).
Ackerman, A. L., Kyritsis, C., Tampe, R. & Cresswell, P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nature Immunol. 6, 107–113 (2005).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Ackerman, A. L., Giodini, A. & Cresswell, P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 25, 607–617 (2006).
Lizee, G. et al. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nature Immunol. 4, 1065–1073 (2003).
den Haan, J. M. & Bevan, M. J. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8+ and CD8− dendritic cells in vivo. J. Exp. Med. 196, 817–827 (2002).
Gallegos, A. M. & Bevan, M. J. Central tolerance: good but imperfect. Immunol. Rev. 209, 290–296 (2006).
Brocker, T., Riedinger, M. & Karjalainen, K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J. Exp. Med. 185, 541–550 (1997).
Gallegos, A. M. & Bevan, M. J. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 200, 1039–1049 (2004). The authors describe a role for cross-presentation of tissue antigens that are ectopically expressed by epithelial cells of the thymus in negative selection of autoreactive thymocytes. They also show that central cross-tolerance is mediated by thymic-resident DCs.
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Belz, G. T. et al. Cutting edge: conventional CD8α+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172, 1996–2000 (2004).
Belz, G. T., Shortman, K., Bevan, M. J. & Heath, W. R. CD8α+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 175, 196–200 (2005).
Neuenhahn, M. et al. CD8α+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity 25, 619–630 (2006).
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nature Immunol. 5, 1143–1148 (2004).
Wykes, M., Keighley, C., Pinzon-Charry, A. & Good, M. F. Dendritic cell biology during malaria. Cell. Microbiol. 9, 300–305 (2007).
Williamson, W. A. & Greenwood, B. M. Impairment of the immune response to vaccination after acute malaria. Lancet 1, 1328–1329 (1978).
Whittle, H. C. et al. T-cell control of Epstein–Barr virus-infected B cells is lost during P. falciparum malaria. Nature 312, 449–450 (1984).
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2002).
Benjamim, C. F., Lundy, S. K., Lukacs, N. W., Hogaboam, C. M. & Kunkel, S. L. Reversal of long-term sepsis-induced immunosuppression by dendritic cells. Blood 105, 3588–3595 (2005).
Yoshino, M. et al. Distinct antigen trafficking from skin in the steady and active states. Int. Immunol. 15, 773–779 (2003).
Scheinecker, C., McHugh, R., Shevach, E. M. & Germain, R. N. Constitutive presentation of a natural tissue autoantigen exclusively by dendritic cells in the draining lymph node. J. Exp. Med. 196, 1079–1090 (2002).
Belz, G. T. et al. The CD8α+ dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 196, 1099–1104 (2002).
Carbone, F. R., Belz, G. T. & Heath, W. R. Transfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunity. Trends Immunol. 25, 655–658 (2004).
Lee, J. W. et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nature Immunol. 8, 181–190 (2007).
Mayerova, D., Parke, E. A., Bursch, L. S., Odumade, O. A. & Hogquist, K. A. Langerhans cells activate naive self-antigen-specific CD8 T cells in the steady state. Immunity 21, 391–400 (2004).
Kissenpfennig, A. & Malissen, B. Langerhans cells — revisiting the paradigm using genetically engineered mice. Trends Immunol. 27, 132–139 (2006).
Filippi, C. et al. CD4+ T cell polarization in mice is modulated by strain-specific major histocompatibility complex-independent differences within dendritic cells. J. Exp. Med. 198, 201–209 (2003).
Lemos, M. P., Esquivel, F., Scott, P. & Laufer, T. M. MHC class II expression restricted to CD8α+ and CD11b+ dendritic cells is sufficient for control of Leishmania major. J. Exp. Med. 199, 725–730 (2004).
Allan, R. S. et al. Epidermal viral immunity induced by CD8α+ dendritic cells but not by Langerhans cells. Science 301, 1925–1928 (2003).
Zhao, X. et al. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197, 153–162 (2003). References 131 and 132 report the surprising finding that Langerhans cells are not involved in induction of T-cell responses against HSV infections of the skin. Instead, resident CD8+ DCs that cross-present viral antigens on MHC class I (reference 131) and dermal DCs that present the antigens on MHC class II (reference 132) are the main DC subsets involved.
Allan, R. S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Belz, G. T. et al. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl Acad. Sci. USA 101, 8670–8675 (2004).
Fleeton, M. N. et al. Peyer's patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J. Exp. Med. 200, 235–245 (2004).
Villadangos, J. A. Hold on, the monocytes are coming! Immunity 26, 390–392 (2007).
Finkelman, F. D., Lees, A., Birnbaum, R., Gause, W. C. & Morris, S. C. Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J. Immunol. 157, 1406–1414 (1996).
Steinman, R. M. & Nussenzweig, M. C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc. Natl Acad. Sci. USA 99, 351–358 (2002).
Acknowledgements
We thank F. Carbone, B. Heath, C. Kurts and K. Shortman for helpful discussions. Our work is supported by grants from the National Health and Medical Research Council of Australia and the Anti-Cancer Council of Australia (J.A.V.), a Leukemia and Lymphoma Society Scholarship (J.A.V.), and a Gottlieb Daimler- and Karl Benz-Foundation Fellowship (P.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Toll-like receptor
-
(TLR). A member of a family of receptors that is homologous to Drosophila melanogaster Toll. TLRs recognize conserved molecular patterns that are unique to microorganisms. The lipopolysaccharide component of bacterial cell walls is one such component. TLRs can also recognize mammalian components and contribute to autoimmunity.
- Nucleotide-binding oligomerization domain (NOD) proteins
-
Members of a family that includes the apoptosis regulator APAF1 (apoptotic-protease-activating factor 1), mammalian NOD-LRR proteins (also known as NACHT-LRR proteins or CATERPILLERs) and plant disease-resistance gene products. Several NOD proteins have been implicated in the induction of nuclear factor-κB activity and in the activation of caspases.
- RIG-I-like receptors
-
A family of cytoplasmic pathogen sensors that recognize viral double-stranded RNA molecules and trigger an antiviral response.
- C-type lectin receptors
-
A large family of receptors that bind glycosylated ligands and have multiple roles, such as in cell adhesion, endocytosis, natural-killer-cell target recognition and dendritic-cell activation.
- Proteasome
-
A giant multicatalytic protease that is resident in the cytosol and the nucleus.
- Cathepsins
-
A class of proteases that are localized mainly in lysosomes and lysosome-like organelles.
- Pinocytosis
-
Also known as fluid-phase endocytosis. A process of engulfment of extracellular fluid and its solutes. It can be mediated by an actin-dependent mechanism that can engulf large volumes (macropinocytosis) or by other mechanisms that result in engulfment of smaller volumes (micropinocytosis).
- Phagocytosis
-
A process that is used by cells to internalize large particles, such as debris, apoptotic cells and pathogens, into phagosomes.
- Negative selection
-
The deletion of self-reactive thymocytes in the thymus. Thymocytes expressing T-cell receptors that strongly recognize self peptide bound to self MHC molecules undergo apoptosis in response to the signalling generated by high-affinity binding.
Rights and permissions
About this article
Cite this article
Villadangos, J., Schnorrer, P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol 7, 543–555 (2007). https://doi.org/10.1038/nri2103
Issue Date:
DOI: https://doi.org/10.1038/nri2103
This article is cited by
-
Incorporating the Molecular Mimicry of Environmental Antigens into the Causality of Autoimmune Hepatitis
Digestive Diseases and Sciences (2023)
-
Metformin enhances the antitumor activity of oncolytic herpes simplex virus HF10 (canerpaturev) in a pancreatic cell cancer subcutaneous model
Scientific Reports (2022)
-
Nanoscale organization of the MHC I peptide-loading complex in human dendritic cells
Cellular and Molecular Life Sciences (2022)
-
Paraneoplastic neurological syndrome: growing spectrum and relevance
Neurological Sciences (2022)
-
Human leukocyte antigen class II quantification by targeted mass spectrometry in dendritic-like cell lines and monocyte-derived dendritic cells
Scientific Reports (2021)