Key Points
-
Haematopoietic stem cells (HSCs) have the clonal capacity to lifelong reconstitute all blood lineages after transplantation into lethally irradiated recipients, and are thought to be localized in a specific microenvironment called the niche.
-
An individual stem cell can give rise to two non-identical daughter cells, one maintaining stem-cell identity and the other becoming a differentiated cell. There are two mechanisms by which this asymmetry can be achieved, depending on whether it occurs pre- (divisional asymmetry), or post- (environmental asymmetry) cell division.
-
Adult stem cells are located in a specific stem-cell-fate-maintaining microenvironment called the niche. A stem-cell niche can be defined as a spatial structure in which stem cells are housed and maintained by allowing self-renewal in the absence of differentiation.
-
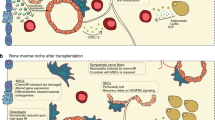
Bone-marrow HSCs and their niches are either located near the endosteal lining of the bone-marrow cavities (the endosteal niche) or are in close contact to the endothelium of the sinusoids (the vascular niche).
-
The endosteal niche comprises specialized osteoblastic cells. The physical interaction between HSCs and osteoblastic cells is mediated by a variety of cell-adhesion molecules including N-cadherin and members of the integrin family.
-
The tight physical interaction between HSCs and niche cells allows molecular crosstalk of ligand–receptor pairs including membrane-bound stem-cell factor (SCF)–KIT, angiopoietin-1 (ANG1)– tyrosine kinase receptor 2 (TIE2) and bone morphogenetic protein (BMP)–BMP-receptor-1A (BMPR1A), which on one hand maintain HSC fate and on the other hand preserve niche activity. We propose the term 'stem-cell–niche synapse' for this adhesion and signalling unit that controls HSC self-renewal and differentiation as well as stem-cell quiescence.
Abstract
Adult stem cells hold many promises for future clinical applications and regenerative medicine. The haematopoietic stem cell (HSC) is the best-characterized somatic stem cell so far, but in vitro expansion has been unsuccessful, limiting the future therapeutic potential of these cells. Here we review recent progress in characterizing the composition of the HSC bone-marrow microenvironment, known as the HSC niche. During homeostasis, HSCs, and therefore putative bone-marrow HSC niches, are located near bone surfaces or are associated with the sinusoidal endothelium. The molecular crosstalk between HSCs and the cellular constituents of these niches is thought to control the balance between HSC self-renewal and differentiation, indicating that future successful expansion of HSCs for therapeutic use will require three-dimensional reconstruction of a stem-cell–niche unit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fuchs, E., Tumbar, T. & Guasch, G. Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 (2004).
Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34−low/negative hematopoietic stem cell. Science 273, 242–245 (1996).
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000).
Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. Cell 100, 157–168 (2000).
Kondo, M. et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21, 759–806 (2003).
Till, J. E. & McCulloch, C. E. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 14, 213–222 (1961).
Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 297, 2256–2259 (2002).
Matsuzaki, Y., Kinjo, K., Mulligan, R. C. & Okano, H. Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity 20, 87–93 (2004).
Kiel, M. J., Yilmaz, O. H., Iwashita, T., Terhorst, C. & Morrison, S. J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 (2005). This study shows that CD150, one of the signalling lymphocytic activation molecules (SLAMs) is expressed by LTR HSCs. CD150+ HSCs were localized near sinusoids in normal bone marrow, indicating that in addition to the endosteal niche a second vascular niche might exist.
Curry, J. L., Trentin, J. J. & Wolf, N. Hemopoietic spleen colony studies. II. Erythropoiesis. J. Exp. Med. 125, 703–720 (1967).
Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). This paper proposes that HSCs are associated with other cell types, and that these allow self-renewal but prevent cell maturation. Schofield termed this microenvironment the 'stem-cell niche'
McCulloch, E. A., Siminovitch, L., Till, J. E., Russell, E. S. & Bernstein, S. E. The cellular basis of the genetically determined hemopoietic defect in anemic mice of genotype Sl-Sld. Blood 26, 399–410 (1965). This paper (together with references 13 and 14) shows that normal bone marrow fails to engraft in Sl/Sld mice. This is probably the first study demonstrating that the microenvironment is essential for bone-marrow HSC function and/or maintenance.
Barker, J. E. Sl/Sld hematopoietic progenitors are deficient in situ. Exp. Hematol. 22, 174–177 (1994).
Barker, J. E. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp. Hematol. 25, 542–547 (1997).
Nilsson, S. K. & Simmons, P. J. Transplantable stem cells: home to specific niches. Curr. Opin. Hematol. 11, 102–106 (2004).
Ohlstein, B., Kai, T., Decotto, E. & Spradling, A. The stem cell niche: theme and variations. Curr. Opin. Cell Biol. 16, 693–699 (2004).
Spradling, A., Drummond-Barbosa, D. & Kai, T. Stem cells find their niche. Nature 414, 98–104 (2001).
Wu, A. M., Siminovitch, L., Till, J. E. & McCulloch, E. A. Evidence for a relationship between mouse hemopoietic stem cells and cells forming colonies in culture. Proc. Natl Acad. Sci. USA 59, 1209–1215 (1968).
Domen, J. & Weissman, I. L. Self-renewal, differentiation or death: regulation and manipulation of hematopoietic stem cell fate. Mol. Med. Today 5, 201–208 (1999).
Yang, L. et al. Identification of Lin−Sca1+kit+CD34+Flt3− short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105, 2717–2723 (2005).
Adolfsson, J. et al. Identification of Flt3+lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121, 295–306 (2005).
Christensen, J. L. & Weissman, I. L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl Acad. Sci. USA 98, 14541–14546 (2001).
Jordan, C. T. & Lemischka, I. R. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 4, 220–232 (1990).
Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C. & Melton, D. A. 'Stemness': transcriptional profiling of embryonic and adult stem cells. Science 298, 597–600 (2002).
Ivanova, N. B. et al. A stem cell molecular signature. Science 298, 601–604 (2002).
Akashi, K. et al. Transcriptional accessibility for genes of multiple tissues and hematopoietic lineages is hierarchically controlled during early hematopoiesis. Blood 101, 383–389 (2003).
Venezia, T. A. et al. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2, e301 (2004).
Roegiers, F. & Jan, Y. N. Asymmetric cell division. Curr. Opin. Cell Biol. 16, 195–205 (2004).
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005). This paper shows that some basal cells of the skin epidermis divide in an asymmetric manner (divisional asymmetry), and that this correlates with the unequal distribution of determinants including PAR3, LGN and mINSC in the two daughter cells.
Suda, J., Suda, T. & Ogawa, M. Analysis of differentiation of mouse hemopoietic stem cells in culture by sequential replating of paired progenitors. Blood 64, 393–399 (1984).
Takano, H., Ema, H., Sudo, K. & Nakauchi, H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J. Exp. Med. 199, 295–302 (2004).
Ho, A. D. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp. Hematol. 33, 1–8 (2005).
Xie, T. & Spradling, A. C. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330 (2000).
Kiger, A. A., White-Cooper, H. & Fuller, M. T. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature 407, 750–754 (2000).
Fleming, W. H. et al. Functional heterogeneity is associated with the cell cycle status of murine hematopoietic stem cells. J. Cell Biol. 122, 897–902 (1993).
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004). In this paper, transgenic expression of H2B–EGFP is used to show that slow-cycling skin epidermal stem cells are primarily located in the hair follicle bulge. This technique, which identifies and allows purification of viable LRCs, should be adaptable for analysis of other adult stem cells and their associated niches.
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003). N-cadherin was proposed as a critical molecule that anchors HSCs to osteoblasts through homotypic interactions. This study (together with reference 67), also shows that osteoblastic cells are a critical component of the endosteal bone-marrow niche.
Lerner, C. & Harrison, D. E. 5-Fluorouracil spares hemopoietic stem cells responsible for long-term repopulation. Exp. Hematol. 18, 114–118 (1990).
Spangrude, G. J. & Johnson, G. R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc. Natl Acad. Sci. USA 87, 7433–7437 (1990).
Uchida, N. et al. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood 89, 465–472 (1997).
Suda, T., Arai, F. & Hirao, A. Hematopoietic stem cells and their niche. Trends Immunol. 26, 426–433 (2005).
Potten, C. S. & Loeffler, M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110, 1001–1020 (1990).
Arai, F. et al. Tie2/angiopoietin-1 signalling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161 (2004). This paper shows that the ANG1 produced by niche osteoblasts activates TIE2, which maintains HSC quiescence and increases N-cadherin expression, and therefore increases adhesion to the endosteal niche.
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001).
Van Zant, G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J. Exp. Med. 159, 679–690 (1984).
Randall, T. D. & Weissman, I. L. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood 89, 3596–3606 (1997).
Uchida, N. et al. ABC transporter activities of murine hematopoietic stem cells vary according to their developmental and activation status. Blood 103, 4487–4495 (2004).
Chambers, I. & Smith, A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23, 7150–7160 (2004).
Cheshier, S. H., Morrison, S. J., Liao, X. & Weissman, I. L. In vivo proliferation and cell cycle kinetics of long-term self- renewing hematopoietic stem cells. Proc. Natl Acad. Sci. USA 96, 3120–3125 (1999).
Wilson, A. et al. c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes Dev. 18, 2747–2763 (2004). This paper provides genetic evidence that differential expression of MYC controls the balance between stem-cell self-renewal and differentiation by regulating entry and exit of HSCs from the endosteal niche.
Murphy, M. J., Wilson, A. & Trumpp, A. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 15, 128–137 (2005).
Patt, H. M. & Maloney, M. A. Bone formation and resorption as a requirement for marrow development. Proc. Soc. Exp. Biol. Med. 140, 205–207 (1972).
Maloney, M. A. & Patt, H. M. On the origin of hematopoietic stem cells after local marrow extirpation. Proc. Soc. Exp. Biol. Med. 149, 94–97 (1975).
Deguchi, K. et al. Excessive extramedullary hematopoiesis in Cbfa1-deficient mice with a congenital lack of bone marrow. Biochem. Biophys. Res. Commun. 255, 352–359 (1999).
Ducy, P., Schinke, T. & Karsenty, G. The osteoblast: a sophisticated fibroblast under central surveillance. Science 289, 1501–1504 (2000).
Karsenty, G. & Wagner, E. F. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell 2, 389–406 (2002).
Lord, B. I., Testa, N. G. & Hendry, J. H. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood 46, 65–72 (1975).
Gong, J. K. Endosteal marrow: a rich source of hematopoietic stem cells. Science 199, 1443–1445 (1978). This study provides the first functional evidence for the location of HSCs and progenitor cells close to endosteal surfaces of rat bone.
Askenasy, N. & Farkas, D. L. In vivo imaging studies of the effect of recipient conditioning, donor cell phenotype and antigen disparity on homing of haematopoietic cells to the bone marrow. Br. J. Haematol. 120, 505–515 (2003).
Sipkins, D. A. et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969–973 (2005).
Nilsson, S. K., Johnston, H. M. & Coverdale, J. A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97, 2293–2299 (2001).
Taichman, R. S. & Emerson, S. G. The role of osteoblasts in the hematopoietic microenvironment. Stem Cells 16, 7–15 (1998).
Taichman, R. S. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105, 2631–2639 (2005).
Oostendorp, R. A. et al. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood 99, 1183–1189 (2002).
Calvi, L. M. et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846 (2003).
Chen, D., Zhao, M. & Mundy, G. R. Bone morphogenetic proteins. Growth Factors 22, 233–241 (2004).
Visnjic, D. et al. Conditional ablation of the osteoblast lineage in Col2.3δk transgenic mice. J. Bone Miner. Res. 16, 2222–2231 (2001).
Visnjic, D. et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264 (2004). This paper shows that conditional ablation of osteoblasts results in a reversible decrease of bone-marrow HSCs and extramedullary haematopoiesis, indicating that osteoblasts are not only required for maintenance of bone-marrow haematopoiesis, but are also an essential component of the niche.
Corral, D. A. et al. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc. Natl Acad. Sci. USA 95, 13835–13840 (1998).
Huber, T. L., Kouskoff, V., Fehling, H. J., Palis, J. & Keller, G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432, 625–630 (2004).
Ohneda, O. et al. Hematopoietic stem cell maintenance and differentiation are supported by embryonic aorta-gonad-mesonephros region-derived endothelium. Blood 92, 908–919 (1998).
Li, W. et al. Primary endothelial cells isolated from the yolk sac and para-aortic splanchnopleura support the expansion of adult marrow stem cells in vitro. Blood 102, 4345–4353 (2003).
Li, W., Johnson, S. A., Shelley, W. C. & Yoder, M. C. Hematopoietic stem cell repopulating ability can be maintained in vitro by some primary endothelial cells. Exp. Hematol. 32, 1226–1237 (2004).
Kopp, H. G., Avecilla, S. T., Hooper, A. T. & Rafii, S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda Md.) 20, 349–356 (2005).
Avecilla, S. T. et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature Med. 10, 64–71 (2004).
Rafii, S., Mohle, R., Shapiro, F., Frey, B. M. & Moore, M. A. Regulation of hematopoiesis by microvascular endothelium. Leuk. Lymphoma 27, 375–386 (1997).
Heissig, B. et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637 (2002).
Rafii, S. et al. Human bone marrow microvascular endothelial cells support long-term proliferation and differentiation of myeloid and megakaryocytic progenitors. Blood 86, 3353–3363 (1995).
Delehanty, L. L. et al. Stromal inhibition of megakaryocytic differentiation is associated with blockade of sustained Rap1 activation. Blood 101, 1744–1751 (2003).
Tokoyoda, K., Egawa, T., Sugiyama, T., Choi, B. I. & Nagasawa, T. Cellular niches controlling B lymphocyte behaviour within bone marrow during development. Immunity 20, 707–718 (2004).
Hirose, J. et al. A developing picture of lymphopoiesis in bone marrow. Immunol. Rev. 189, 28–40 (2002).
Papayannopoulou, T. Bone marrow homing: the players, the playfield, and their evolving roles. Curr. Opin. Hematol. 10, 214–219 (2003).
Lapidot, T., Dar, A. & Kollet, O. How do stem cells find their way home? Blood 106, 1901–1910 (2005).
Cancelas, J. A. et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nature Med. 11, 886–891 (2005).
Lapidot, T. & Petit, I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30, 973–981 (2002).
Wright, D. E., Wagers, A. J., Gulati, A. P., Johnson, F. L. & Weissman, I. L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
Potocnik, A. J., Brakebusch, C. & Fassler, R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12, 653–663 (2000).
Nilsson, S. K. et al. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood 101, 856–862 (2003).
Nilsson, S. K. et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239 (2005).
Ara, T. et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 (2003). The authors show that CXCL12 has an important role in colonization of the bone marrow by HSCs.
Ponomaryov, T. et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Invest. 106, 1331–1339 (2000).
Wright, D. E., Bowman, E. P., Wagers, A. J., Butcher, E. C. & Weissman, I. L. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J. Exp. Med. 195, 1145–1154 (2002).
Nagasawa, T. et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638 (1996).
Zou, Y. R., Kottmann, A. H., Kuroda, M., Taniuchi, I. & Littman, D. R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Gu, Y. et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445–449 (2003).
Moore, K. A. Recent advances in defining the hematopoietic stem cell niche. Curr. Opin. Hematol. 11, 107–111 (2004).
Artavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signalling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Radtke, F., Wilson, A., Mancini, S. J. & MacDonald, H. R. Notch regulation of lymphocyte development and function. Nature Immunol. 5, 247–253 (2004).
Duncan, A. W. et al. Integration of Notch and Wnt signalling in hematopoietic stem cell maintenance. Nature Immunol. 6, 314–322 (2005).
Varnum-Finney, B. et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signalling. Nature Med. 6, 1278–1281 (2000).
Stier, S., Cheng, T., Dombkowski, D., Carlesso, N. & Scadden, D. T. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favours lymphoid over myeloid lineage outcome. Blood 99, 2369–2378 (2002).
Han, H. et al. Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int. Immunol. 14, 637–645 (2002).
Mancini, S. J. et al. Jagged1-dependent Notch signalling is dispensable for hematopoietic stem cell self-renewal and differentiation. Blood 105, 2340–2342 (2005).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Saito, T. et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 18, 675–685 (2003).
Denhardt, D. T. & Guo, X. Osteopontin: a protein with diverse functions. FASEB J. 7, 1475–1482 (1993).
Stier, S. et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J. Exp. Med. 201, 1781–1791 (2005).
Flanagan, J. G., Chan, D. C. & Leder, P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell 64, 1025–1035 (1991).
Lyman, S. D. & Jacobsen, S. E. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 91, 1101–1134 (1998).
Miyazawa, K. et al. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood 85, 641–649 (1995).
Kinashi, T. & Springer, T. A. Steel factor and c-kit regulate cell-matrix adhesion. Blood 83, 1033–1038 (1994).
Kovach, N. L., Lin, N., Yednock, T., Harlan, J. M. & Broudy, V. C. Stem cell factor modulates avidity of α4 β1 and α5 β1 integrins expressed on hematopoietic cell lines. Blood 85, 159–167 (1995).
Driessen, R. L., Johnston, H. M. & Nilsson, S. K. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp. Hematol. 31, 1284–1291 (2003).
Lotinun, S., Evans, G. L., Turner, R. T. & Oursler, M. J. Deletion of membrane-bound steel factor results in osteopenia in mice. J. Bone Miner. Res. 20, 644–652 (2005).
Radice, G. L. et al. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 181, 64–78 (1997).
Puri, M. C. & Bernstein, A. Requirement for the TIE family of receptor tyrosine kinases in adult but not fetal hematopoiesis. Proc. Natl Acad. Sci. USA 100, 12753–12758 (2003).
Cheng, T. et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000).
Cheng, T., Shen, H., Rodrigues, N., Stier, S. & Scadden, D. T. Transforming growth factor β1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21(Cip1/Waf1) or p27(Kip1). Blood 98, 3643–3649 (2001).
Wu, S. et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene 22, 351–360 (2003).
Reiss, K. et al. ADAM10 cleavage of N-cadherin and regulation of cell–cell adhesion and β-catenin nuclear signalling. EMBO J. 24, 742–752 (2005).
Ito, K. et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of β-catenin from cell–cell contacts. Oncogene 18, 7080–7090 (1999).
Willert, K. et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423, 448–452 (2003). This paper, together with reference 126, provides evidence of a positive role for WNT signalling in HSC self-renewal, whereas differentiation (at least in vitro ) is inhibited.
Reya, T. et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 423, 409–414 (2003).
Cobas, M. et al. β-catenin is dispensable for hematopoiesis and lymphopoiesis. J. Exp. Med. 199, 221–229 (2004).
Friedl, P. & Storim, J. Diversity in immune-cell interactions: states and functions of the immunological synapse. Trends Cell Biol. 14, 557–567 (2004).
Powell, K. It's the ecology, stupid. Nature 435, 268–270 (2005).
Sorrentino, B. P. Clinical strategies for expansion of haematopoietic stem cells. Nature Rev. Immunol. 4, 878–888 (2004).
Ploemacher, R. E. Stem cells: characterization and measurement. Baillieres Clin. Haematol. 10, 429–444 (1997).
Morrison, S. J., Uchida, N. & Weissman, I. L. The biology of hematopoietic stem cells. Annu. Rev. Cell Dev. Biol. 11, 35–71 (1995).
Visser, J. W., Bauman, J. G., Mulder, A. H., Eliason, J. F. & de Leeuw, A. M. Isolation of murine pluripotent hemopoietic stem cells. J. Exp. Med. 159, 1576–1590 (1984).
Bunting, K. D. ABC Transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20, 11–20 (2002).
Goodell, M. A., McKinney-Freeman, S. & Camargo, F. D. Isolation and characterization of side population cells. Methods Mol. Biol. 290, 343–352 (2005).
Uchida, N., Dykstra, B., Lyons, K. J., Leung, F. Y. & Eaves, C. J. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp. Hematol. 31, 1338–1347 (2003).
Camargo, F. D., Chambers, S. M., Drew, E., McNagny, K. M. & Goodell, M. A. Hematopoietic stem cells do not engraft with absolute efficiencies. Blood 107, 501–507 (2006).
Bradford, G. B., Williams, B., Rossi, R. & Bertoncello, I. Quiescence, cycling, and turnover in the primitive hematopoietic stem cell compartment. Exp. Hematol. 25, 445–453 (1997).
Komori, T. et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997).
Otto, F. et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 (1997).
Adams, G. D. et al. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 28 Dec 2005 (doi:10.1038/nature04247).
Acknowledgements
We would like to thank F. Radtke, R. MacDonald, M. Murphy and G. Oser for their critical reading of the manuscript, and members of the Trumpp Laboratory for helpful discussions. We apologize to colleagues whose work could not be cited due to space limitations. This work was in part supported by grants to A.T. from the Swiss National Science Foundation, the Swiss Cancer League and the UBS Optimus Foundation. A.T. is member of the EMBO Young Investigator Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Self-renewal
-
The capacity of a stem cell to divide in such a way that one or both daughter cells retain the stem-cell fate.
- Steel-Dickie mice
-
(Sl/Sld). A spontaneous mouse mutant with a defect in the production of membrane-bound stem-cell factor (SCF), although secreted SCF is produced normally
- BrdU labelling
-
Incorporation of bromodeoxyuridine (BrdU) into newly synthesized DNA permits indirect detection of proliferating cells using fluorescently labelled BrdU-specific antibodies by either flow cytometry or fluorescence microscopy.
- Trabecular bone
-
Also known as cancellous bone, this is found in areas of rapid turnover such as the ends of the long bones.
- Myeloablative agents
-
Used to completely or partially eliminate the haematopoietic system. These agents include the use of whole-body irradiation or cytotoxic drugs such as 5-fluorouracil.
- Osteoblasts
-
Mesenchymal cells that produce bone matrix that forms bone after mineralization.
- Osteoclasts
-
Large, multi-nucleated cells derived from macrophages that resorb bone. The activity of osteoblasts and osteoclasts form an equilibrium that maintains bone during homeostasis and remodelling.
- Endosteum
-
The cellular lining separating bone from bone marrow. It comprises different cell types including osteoblasts, osteoclasts and stromal fibroblasts.
- Bone-marrow sinusoids
-
Low-pressure vascular channels surrounded by a single layer of fenestrated endothelium.
- Bone morphogenetic protein
-
Induces the formation of bone and cartilage, and is a member of the transforming growth factor-β (TGFβ) superfamily.
- Stromal fibroblasts
-
Part of the endosteal lining separating bone and bone marrow.
- Mobilization
-
The efflux of haematopoietic stem cells from the bone marrow into the vasculature in response to bone-marrow stress or injury, or after treatment with cytokines such as granulocyte colony-stimulating factor (G-CSF).
- Homing
-
The specific movement or migration of haematopoietic stem cells through the vasculature to the bone marrow.
- Engraftment
-
The production of more haematopoietic stem cells by symmetrical divisions and production of a large number of progenitors and differentiated cell types.
- LRC assay
-
(Label retaining cell assay). Identifies long-lived quiescent cells such as adult stem cells. They can be visualized in situ by pulse labelling of their DNA with BrdU (or 3H-thymidine or a histone H2B–EGFP transgene) followed by a chase period of a month or more. Detection of BrdU+ cells requires fixation, precluding subsequent functional analysis.
- Angiogenic factors
-
These factors (which include angiopoietin-1) promote the development of blood vessels, and are particularly important in embryonic and fetal development.
- OP9 stromal cells
-
A bone-marrow-derived cell line that can support the expansion of haematopoietic-cell lineages in culture.
Rights and permissions
About this article
Cite this article
Wilson, A., Trumpp, A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6, 93–106 (2006). https://doi.org/10.1038/nri1779
Issue Date:
DOI: https://doi.org/10.1038/nri1779
This article is cited by
-
Mesenchymal stem/stromal cells from human pluripotent stem cell-derived brain organoid enhance the ex vivo expansion and maintenance of hematopoietic stem/progenitor cells
Stem Cell Research & Therapy (2024)
-
Mesenchymal stromal cell-associated migrasomes: a new source of chemoattractant for cells of hematopoietic origin
Cell Communication and Signaling (2023)
-
Understanding Hematopoietic Stem Cell Dynamics—Insights from Mathematical Modelling
Current Stem Cell Reports (2023)
-
The regenerative effect of stem cells on acetaminophen-induced hepatotoxicity in male albino rats
Egyptian Liver Journal (2022)
-
BAP1 shapes the bone marrow niche for lymphopoiesis by fine-tuning epigenetic profiles in endosteal mesenchymal stromal cells
Cell Death & Differentiation (2022)