Abstract
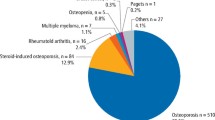
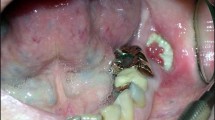
Bisphosphonates are a valuable class of drugs with potent anti-resorptive actions that make them ideal for skeletal protection in osteoporosis, cancer bone metastasis, multiple myeloma, and Paget's disease of bone. It has become apparent, however, that these drugs also have the potential to cause a number of adverse effects. While these do not limit bisphosphonate use, the incidence of these adverse events can be minimized if appropriate care is taken with their administration, and by maintaining appropriate surveillance and patient care. We review the range of adverse reactions to bisphosphonate therapy with a particular emphasis on the recently identified association between long-term bisphosphonate treatment and osteonecrosis of the jaw. This is a potentially serious side effect seen mostly in patients with multiple myeloma or breast cancer bone metastases who receive intravenous bisphosphonate treatment. While the etiology is uncertain, a strong association with dental pathology and interventions highlights the need for close attention to dental health in this patient group.
Key Points
-
Bisphosphonates have proven highly beneficial for treatment of diseases characterized by a relative excess in osteoclastic bone resorption
-
Bisphosphonate treatment can reduce fracture risk in osteoporosis, bone pain in Paget's disease of bone, and hypercalcemic and skeletal events in patients with bone metastases
-
Bisphosphonate treatment has, however, been associated with a number of adverse effects including gastrointestinal injury, nephrotoxicity and, more recently, ONJ
-
These adverse effects can be minimized by awareness of risks, care in drug administration, prophylactic dental care, and careful patient monitoring
-
There is an urgent need to understand the etiology of ONJ to improve evaluation of patient risk and to enable the identification of appropriate treatments
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Liberman UA et al. (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333: 1437–1443
Black DM et al. (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348: 1535–1541
Cummings SR et al. (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280: 2077–2082
Pols HA et al. (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Foxamax International Trial Study Group. Osteoporos Int 9: 461–468
Reginster J et al. (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11: 83–91
Harris ST et al. (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282: 1344–1352
Roux C et al. (2004) Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin 20: 433–439
Felsenberg D et al. (2005) Oral ibandronate significantly reduces the risk of vertebral fractures of greater severity after 1, 2, and 3 years in postmenopausal women with osteoporosis. Bone 37: 651–654
Miller PD et al. (2005) Monthly oral ibandronate therapy in postmenopausal osteoporosis: 1-year results from the MOBILE study. J Bone Miner Res 20: 1315–1322
McClung MR et al. (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344: 333–340
Reid IR et al. (2005) Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med 353: 898–908
Ross JR et al. (2003) Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ 327: 469–472
Jagdev SP et al. (2001) Comparison of the effects of intravenous pamidronate and oral clodronate on symptoms and bone resorption in patients with metastatic bone disease. Ann Oncol 12: 1433–1438
Berenson JR et al. (2001) A phase I dose-ranging trial of monthly infusions of zoledronic acid for the treatment of osteolytic bone metastases. Clin Cancer Res 7: 478–485
Glorieux FH et al. (1998) Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med 339: 947–952
Astrom E and Soderhall S (2002) Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child 86: 356–364
Fleisch H et al. (1969) Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture and in vivo. Science 165: 1262–1264
Rogers MJ et al. (2000) Cellular and molecular mechanisms of action of bisphosphonates. Cancer 88 (Suppl 12): 2961–2978
Lin JH (1996) Bisphosphonates: a review of their pharmacokinetic properties. Bone 18: 75–85
Russell RG et al. (1970) The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif Tissue Res 6: 183–196
Schenk R et al. (1973) Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichloromethylene diphosphonate (Cl 2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif Tissue Int 11: 326–331
Thompson K et al. (2006) Cytosolic entry of bisphosphonate drugs requires acidification of vesicles after fluid-phase endocytosis. Mol Pharmacol 69: 1624–1632
Sato M et al. (1991) Bisphosphonate action: alendronate localization in rat bone osteoclast ultrastructure. J Clin Invest 88: 2095–2105
Van Rooijen N et al. (1990) Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res 260: 215–222
Rogers MJ (2003) New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des 9: 2643–2658
Evans RA et al. (1983) Pathologic fracture due to severe osteomalacia following diphosphonate treatment of Paget's disease of bone. Aust NZ J Med 13: 277–279
Boyce BF et al. (1984) Focal osteomalacia due to low-dose diphosphonate therapy in disease. Lancet 1: 821–824
Boyce BF et al. (1994) Mineralisation defects after pamidronate for Paget's. Lancet 343: 1231–1232
Harris S et al. (1982) Secondary hyperparathyroidism associated with dichloromethane diphosphonate treatment of Paget's disease. J Clin Endocrinol Metab 55: 1100–1107
Champallou C et al. (2003) Hypocalcemia following pamidronate administration for bone solid tumor: three clinical case reports. J Pain Symptom Manage 25: 185–190
Jones SG et al. (2002) Severe increase in creatinine with hypocalcaemia in myeloma patients receiving zoledronic acid infusions. Br J Haematol 119: 576–577
McIntyre E and Bruera E (1996) Symptomatic hypocalcemia after intravenous pamidronate. J Palliative Care 12: 46–47
Mishra A et al. (2001) Prolonged, symptomatic hypocalcemia with pamidronate subclinical hypoparathyroidism. Endocrine 14: 159–164
Haworth CS et al. (1998) Severe bone pain after intravenous pamidronate in adult cystic fibrosis. Lancet 352: 1753–1754
Duarte M et al. (1998) The spectrum of bone disease in 200 chronic hemodialysis patients: a correlation between clinical, biochemical and histological findings. Sao Paulo Med J 116: 1790–1797
Mashiba T et al. (2001) Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone 28: 524–531
Bone H et al. (2004) Alendronate Phase III Osteoporosis Treatment Study Group. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350: 1189–1199
Parfitt A (2003) Renal bone disease: a new conceptual framework for the interpretation of bone histomorphometry. Curr Op Nephrol Hypertension 12: 3787–3403
Odvina CV et al. (2001) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90: 1294–1301
Hewitt RE et al. (2005) The bisphosphonate acute phase response: rapid and copious production of proinflammatory cytokines by peripheral blood gd T cells in response to aminobisphosphonates is inhibited by statins. Clin Exp Immunol 139: 101–111
Thompson K and Rogers MJ (2004) Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res 19: 278–288
Adami S et al. (1987) The acute-phase response after bisphosphonate. Calcif Tissue Int 41: 326–331
Reszka AA et al. (2001) Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol Pharmacol 59: 193–202
Lichtenberger L et al. (2000) Effect of bisphosphonates on surface hydrophobicity and phosphatidylcholine concentration of rodent gastric mucosa. Dig Dis Sci 45: 1792–1801
de Groen PC et al. (1996) Esophagitis associated with the use of alendronate. N Engl J Med 335: 1016–1021
Demerjian N et al. (1999) Severe oral ulcerations induced by alendronate. Clin Rheumatol 18: 349–350
Graham DY and Malaty HM (1999) Alendronate gastric ulcers. Aliment Pharmacol Ther 13: 515–519
De S et al. (1995) Pamidronate and uveitis. Br J Rheumatol 34: 479
Durnian JM et al. (2005) Bilateral acute uveitis and conjunctivitis after zoledronic therapy. Eye 19: 221–222
Fraunfelder FW and Fraunfelder FT (2003) Bisphosphonates and ocular inflammation. N Engl J Med 348: 1187–1188
Bounameaux HM et al. (1983) Renal failure associated with intravenous diphosphonates. Lancet 1: 471
Chang JT et al. (2003) Renal failure with the use of zoledronic acid. N Engl J Med 349: 1676–1679
Markowitz GS et al. (2001) Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172
Munier A et al. (2005) Zoledronic acid and renal toxicity: data from French adverse effect reporting database. Ann Pharmacother 39: 1194–1197
Rosen LS et al. (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7: 377–387
Markowitz GS et al. (2003) Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int 64: 281–289
Marx R et al. (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaw: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63: 1567–1575
Wang J et al. (2003) Osteonecrosis of the jaws associated with cancer chemotherapy. J Oral Maxillofac Surg 61: 1104–1107
Ruggiero SL et al. (2004) Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg 62: 527–534
Tarassoff P and Csermak K (2003) Avascular necrosis of the jaws: risk factors in metastatic cancer patients. J Oral Maxillofac Surg 61: 1238–1239
Carter G et al. (2001) Bisphosphonates and avascular necrosis of the jaw. Med J Aust 182: 413–415
Bamias A et al. (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23: 8580–8587
Durie BG et al. (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353: 99–102
Lenz JH et al. (2005) Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates? Craniomaxillofac Surg 33: 395–403
Sung EC et al. (2002) Osteonecrosis of the maxilla as a complication to chemotherapy: a case report. Spec Care Dentist 22: 142–146
Schwartz HC (1982) Osteonecrosis of the jaws: a complication of cancer chemotherapy. Head Neck Surg 4: 251–253
Manolagas S and Weinstein R (1999) New developments in the pathogenesis and treatment of steroid-induced osteoporosis J Bone Miner Res 14: 1061–1066
Frost HM (1960) In vivo osteocyte death. J Bone Joint Surg 42A: 138–143
Cowan J et al. (1995) Glucocorticoid therapy for myasthenia gravis resulting in resorption of the mandibular condyles. J Oral Maxillofac Surg 53: 1091–1096
Anderson G et al. (2006) Thalidomide derivative CC-4047 inhibits osteoclast formation by down regulation of PU.1. Blood 107: 3098–3105
Crane E and List A (2005) Immunomodulatory drugs. Cancer Invest 23: 625–634
Ficarra G et al. (2005) Osteonecrosis of the jaws in periodontal patients with a history of bisphosphonates treatment. J Clin Periodontol 32: 1123–1128
Epstein J et al. (1997) Postradiation osteonecrosis of the mandible: a long-term follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 83: 657–662
Migliorati CA (2003) Bisphosphonates and oral cavity avascular bone necrosis. J Clin Oncol 21: 4253–4254
Wooltorton E (2005) Patients receiving intravenous bisphosphonates should avoid invasive dental procedures. CMAJ 172: 1684
Melo MD and Obeid G (2005) Osteonecrosis of the jaws in patients with a history of receiving bisphosphonate therapy: strategies for prevention and early recognition. J Am Dent Assoc 136: 1675–1681
Plotkin LI et al. (2005) Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of extracellular signal-regulated kinase activation. J Biol Chem 280: 7317–7325
Bekker PJ et al. (2004) A single dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to nuclear factor-B ligand (RANKL), in postmenopausal women. J Bone Miner Res 19: 1059–1066
Murphy MG et al. (2005) Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab 90: 2022–2028
Wood J et al. (2002) Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther 302: 1055–1061
Santini D et al. (2002) Pamidronate induces modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res 8: 1080–1084
Vincenzi B et al. (2003) Bisphosphonates: new antiangiogenic molecules in cancer treatment? Ann Oncol 14: 806–807
Bartl R et al. (2006) Bisphosphonate. [German] In Bisphosphonat-Manual, 83–88 (Eds Bartl R et al.) New York: Springer Berlin-Heidelberg
Abu-Id MH et al. (2006) Bisphosphonate-associated osteonecrosis of the jaw [German]. Mund Kiefer Gesichtschir 10: 73–81
Badros A et al. (2006) Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol 24: 945–952
Bagan JV et al. (2005) Avascular jaw osteonecrosis in association cancer chemotherapy: series of 10 cases. Med Oral Patol Oral Cir Bucal 10 (Suppl 2): E88–E91
Bagan JV et al. (2005) Jaw osteonecrosis associated with bisphosphonates: multiple exposed areas and its relationship to teeth extractions—study of 20 cases. Oral Oncol 42: 327–329
Farrugia MC et al. (2006) Osteonecrosis of the mandible or maxilla associated with the use of new generation bisphosphonates. Laryngoscope 116: 115–120
Gibbs SD et al. (2005) Bisphosphonate-induced osteonecrosis of the jaw requires early detection and intervention. Med J Aust 183: 549–550
Guarneri V et al. (2005) Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 years. Oncologist 10: 842–848
Hoefert S and Eufinger H (2005) Kieferknochennekrosen als mögliche unerwünschte Wirkung von Bisphosphonaten [German]. Mund Kiefer Gesichts Chir 9: 233–238
Hoff A et al. (2005) Osteonecrosis of the jaw in patients receiving intravenous bisphosphonate therapy [abstract #1218]. J Bone Miner Res 20 (Suppl 1): S55
Junod AF et al. (2005) Osteonecrosis of the jaws and bisphosphonates [French]. Rev Med Suisse 1: 2537–2540
Lugassy G et al. (2004) Severe osteomyelitis of the jaw in long-term survivors of multiple myeloma: a new clinical entity. Am J Med 117: 440–441
Maerevoet M et al. (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353: 99–102
Markiewicz MR et al. (2005) Bisphosphonate-associated osteonecrosis of the jaws: a review of current knowledge. J Am Dent Assoc 136: 1669–1674
Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61: 1115–1118
Merigo E et al. (2005) Jawbone necrosis without previous dental extractions associated with the use of bisphosphonates (pamidronate and zoledronate): a four-case report. J Oral Pathol Med 34: 613–617
Najm SA et al. (2005) Bisphosphonates-related jaw osteonecrosis [French]. Presse Med 34: 1073–1077
Pastor-Zuazaga D et al. (2006) Osteonecrosis of the jaws and bisphosphonates: report of three cases. Med Oral Patol Oral Cir Bucal 11: E76–E79
Purcell PM and Boyd IW (2005) Bisphosphonates and osteonecrosis of the jaw. Med J Aust 182: 417–418
Sanna G et al. (2005) Jaw avascular bone necrosis associated with long-term use of bisphosphonates. Ann Oncol 16: 1207–1208
Schirmer I et al. (2005) Bisphosphonates and osteonecrosis of the jaw [German]. Mund Kiefer Gesichtschir 9: 239–245
Schwartz HC (2004) Osteonecrosis and bisphosphonates: correlation versus causation. J Oral Maxillofac Surg 62: 763–764
Zarychanski R et al. (2006) Osteonecrosis of the jaw associated with pamidronate therapy. Am J Hematol 81: 73–75
Acknowledgements
Dr Julie M Brown assisted in manuscript preparation. Dr Colin R Dunstan receives funding from the Government of New South Wales (BioFirst Award) and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dunstan, C., Felsenberg, D. & Seibel, M. Therapy Insight: the risks and benefits of bisphosphonates for the treatment of tumor-induced bone disease. Nat Rev Clin Oncol 4, 42–55 (2007). https://doi.org/10.1038/ncponc0688
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0688
This article is cited by
-
3D porous polyurethane (PU)/ triethanolamine modified hydroxyapatite (TEA-HA) nano composite for enhanced bioactivity for biomedical applications
Journal of Polymer Research (2022)
-
Long-term outcomes of prolonged bisphosphonates more than 2 years in bone metastatic breast cancer: risk vs benefit
Irish Journal of Medical Science (1971 -) (2020)
-
Preparation and characterization of aliphatic polyurethane and modified hydroxyapatite composites for bone tissue engineering
Polymer Bulletin (2020)
-
Simple 3,4-Dihydroxy-L-Phenylalanine Surface Modification Enhances Titanium Implant Osseointegration in Ovariectomized Rats
Scientific Reports (2017)
-
Safety of long-term denosumab therapy: results from the open label extension phase of two phase 3 studies in patients with metastatic breast and prostate cancer
Supportive Care in Cancer (2016)