Abstract
Dual time point imaging (DTPI) and delayed time point imaging have been used for the differentiation of inflammatory and malignant processes and found to enhance the specificity of FDG PET imaging for diagnostic and prognostic purposes. It has been shown that the degree of FDG uptake at the second acquisition time point after the baseline scan increases in malignant cells; in inflammatory or infectious disorders, on the other hand, FDG uptake decreases or remains unchanged at the second time point. Many groups have investigated the application of DTPI and its potential and limitations have been discussed in detail with reference to a wide variety of malignant diseases, including those of the lung. The aim of this review is to describe the role of DTPI to assess both normal and disease states.
Similar content being viewed by others
Introduction
In the last two decades 18F-FDG PET/CT, a powerful modality able to characterize cancer biology, has made major contributions to the practice of oncology, specifically in disease staging and restaging and in the monitoring of treatment in many malignancies [1]. The accumulation of FDG in cells, following its phosphorylation to FDG-6-phosphate by hexokinase, is facilitated by the glycolytic pathway (Fig. 1). This biological pathway allows the characterization of tumor biology and also makes it possible to differentiate malignant cells from normal and inflammatory cells. However, it has now been demonstrated that both inflammatory and infectious disorders have increased glycolytic activity and therefore can mimic malignancy in many settings [2]. Dual time point imaging (DTPI) and delayed time point imaging have been used as two means of differentiating between these two different processes and have thus enhanced the specificity of FDG PET imaging for diagnostic purposes (Fig. 2) [3–14].
The figure above schematically demonstrates the fate of FDG after it enters the cell via cell membrane glucose transporters. Immediately after entering the cell, FDG is phosphorylated to FDG-6-phosphate by hexokinase and, unlike the glucose molecule, cannot be metabolized further. However the fate of FDG-6-phosphate differs in malignant and inflammatory as well as in normal cells. Cancer cells either lack or have low levels of glucose-6-phosphtase and, therefore, FDG-6-phosphate accumulates in the cell continuously over time. In contrast, inflammatory cells are known to have substantial levels of this enzyme, which metabolizes FGD-6-phosphate, and the free FDG released can no longer be retained in the cells and therefore returns to the bloodstream (color figure online)
Time course of FDG accumulation in malignant and inflammatory cells differs as depicted above. While both are shown to incorporate this radiotracer for a period of time, the pattern is substantially different between the two at later time points. Malignant cells continue to show increasing levels of FDG uptake over time. In contrast, inflammatory lesions either reveal a decline in FDG uptake or plateau off after a certain time course. The decline or plateau is likely due to loss of FDG from the cell following metabolization of FDG-6-phosphate by glucose-6-phosphatase. In malignant cells, on the other hand, because the glucose-6-phosphatase is lacking, FDG-6-phosphate is continuously retained and increases over time. We should point out that blood pool activity decreases exponentially soon after the administration of this compound and therefore, the contrast between target tissues (cancer, inflammation, etc.) increases substantially over time and the lesion becomes more distinct. This phenomenon is more prominent for cancer tissues compared with inflammatory lesions (color figure online)
Through in vitro and in vivo imaging experiments, it has been shown that the degree of FDG uptake at the second acquisition time point after the baseline scan increases in malignant cells; in inflammatory or infectious disorders, on the other hand, FDG uptake decreases or remains unchanged at the second time point [2]. The extent of FDG uptake and its clearance depend on the time delay between injection of FDG and the acquisition of images of the disease sites. The cells of highly glycolytic tissues continuously trap FDG in the form of FDG-6-phosphate, which may remain intact or be dephosphorylated by the enzyme glucose-6-phosphatase inside the cell. It has been speculated that the level of glucose-6-phosphatase is one of the factors that, through DTPI, make it possible to differentiate malignant from benign lesions [15–17]. Cancer cells likely contain low levels of glucose-6-phosphatase for dephosphorylation of FDG-6-phosphate and this could explain the continuous accumulation of FDG-6-phosphate in malignant cells, revealed on second time point images [16, 17]; the opposite occurs in inflammation and infection, due to high levels of glucose-6-phosphatase. It is hypothesized that free FDG, after being separated from phosphate, will leave the cell and become detectable by delayed time point imaging [15]. In addition, high levels of glucose transporters and hexokinase in malignant cells contribute to significant accumulation of FDG in cancer cells over time [2, 18, 19].
The application of DTPI has been investigated by many groups and its potential and limitations [2] have been discussed in detail with reference to a wide variety of malignant diseases including those of the lung [6, 20–24], breast [7, 11, 25–28], head and neck [8, 29], colorectal region [30, 31], brain [32, 33], and lymphatic tissues [3, 34], as well as pediatric cancers [35], gallbladder carcinoma [36]) as well as nonmalignant disorders (atherosclerosis [37, 38], inflammation [39]) and normal states [40, 41] (Table 1). The aim of this review is to describe the role of DTPI in assessing both normal and disease states.
Normal states: dynamic changes in normal tissues
The degree of FDG uptake and its retention in the cells is highly dependent on a multitude of factors, including tracer distribution time and plasma glucose levels [42]. In addition, the background activity decreases over time with DTPI, therefore the contrast between the target lesion and the surrounding tissues increases [2]. For this reason, standardized uptake values (SUVs) vary depending on the interval that elapses between the administration of FDG and the image acquisition. Currently, no specific time point has been adopted for differentiating benign disorders from malignant diseases. Therefore, to properly interpret PET images, it is essential to have some specific knowledge about the dynamics of FDG at different time points [12, 41].
Normal tissues have different metabolic rates and glycolytic activities and, as in pathological states, the levels of glucose-6-phosphatase and of glucose transporters and hexokinase dictate the dynamics of FDG in various normal tissues. Accordingly, an understanding of the physiological uptake levels of each tissue type becomes crucial in order to employ appropriate imaging protocols, and particularly to implement DTPI for differentiation of malignant from benign lesions [12, 41].
Cheng et al. [41] determined FDG uptake and clearance in normal tissues in 30 patients examined by PET at 1, 2 and 3 h after the administration of FDG; to do this they measured the SUVmax and SUVmean of various normal tissues. The results of this study revealed that blood pool, liver and spleen FDG levels decreased from the first to the second hour and from the second to the third hour, while those of the lungs, pancreas, lymph node and skeletal muscles decreased only between the first and second hour. In contrast, bone marrow FDG uptake values were found to be increased on delayed images. The parotid gland, thyroid gland and prostate did not show any significant changes on delayed imaging. In another study by the same authors, it was further shown that FDG uptake values in the left ventricle increased with delayed time point imaging. This study also confirmed finding of the previous study concerning disproportional degrees of increased FDG uptake in the areas of myocardium with a higher SUVmax on the initial scan [40].
Basu et al. [43] prospectively investigated the temporal profile of FDG uptake over periods of up to 8 h in normal tissues as well as in cancerous lesions and reported a trend towards a steady rise in the SUVs of malignant lesions in this time frame, while the SUVs of the normal organs stayed the same or decreased. The authors concluded that delayed imaging over time improves the sensitivity of FDG PET for detecting malignant lesions. They also observed varying slopes of FDG uptake over time, which they interpreted as reflecting tumor heterogeneity and the underlying tumor biology of the lesions examined.
Differentiating benign from malignant lesions
As noted above, the accumulation of FDG is dependent on many factors. Among these, glucose-6-phosphatase, hexokinase and GLUT transporters appear to be critical ones and should therefore, by means of DTPI, be characterized on the basis of their capacity to differentiate between benign and malignant lesions. Brain and heart tissues both show high levels of FDG uptake, likely due to high levels of GLUT transporters and relatively low levels of glucose-6-phosphatase. Malignant cells also possess considerable numbers of GLUT transporters with a decreased ratio of glucose-6-phosphatase to hexokinase, leading to substantial degrees of FDG accumulation. Different types of malignant cells have variable concentrations of glucose-6-phosphatase and therefore show variable time-activity FDG uptake curves. In contrast, it has been speculated that inflammatory cells have higher levels of glucose-6-phosphatase with an increased ratio of glucose-6-phosphatase to hexokinase, which results in breakdown of FDG-6-phosphate and clearance of FDG from the cells over time [44].
Nonmalignant diseases
Atherosclerosis
Delayed time point imaging has been used for the visualization of atherosclerotic plaques. Blomberg et al. [37] in their study, determined the ideal time point, following the administration of FDG, for detecting and quantifying the presence and degree of atherosclerotic plaque inflammation by FDG PET/CT. They imaged 15 patients at three time points (1, 2, and 3 h post injection) and assessed aortic and carotid FDG uptake using qualitative and semi-quantitative methods. They noted significantly improved visualization of atherosclerotic plaques on the delayed images. The aortic and carotid mean target-to-background ratios (TBRs) at the first hour were 1.05 (95 % CI 0.98, 1.11) and 0.88 (95 % CI 0.81, 0.96), respectively. At the third hour, they rose to 1.57 (95 % CI 1.28, 1.86; p = 0.001) and 1.61 (95 % CI 1.36, 1.87; p < 0.001), respectively.
In another study, Blomberg et al. [38] employed DTPI in a prospective study of 40 subjects using FDG PET/CT. FDG parameters were measured on 1.5 and 3 h scans and the results were compared with 10-year risk for fatal cardiovascular disease (SCORE %). The authors found significant increases in the FDG uptake parameters over time in both carotid arteries and in the aorta. The correlation with cardiovascular risk was not significant at the first time point but a significant correlation between the corrected SUVmax of the carotid arteries (τ = 0.25, p = 0.045) and aorta (τ = 0.33, p = 0.008) and SCORE % was found at the second time point (3 h). The authors therefore concluded that delayed time point imaging improves the quantification of atherosclerosis and allows accurate assessment of this major cardiovascular risk factor. These findings demonstrate that over time, with declining background blood pool activity, the contrast between target tissues improves regardless of their underlying disease process (cancer, inflammation, etc.), and the sensitivity of FDG PET in detecting various abnormalities in many organs increases.
Infectious/inflammatory lung diseases
The role of DTPI has been tested in settings other than those of distinguishing malignant from benign disorders. Umeda et al. [45] assessed differential diagnosis and prediction of disease activity in patients with idiopathic interstitial pneumonitis (IIP). They scanned 50 patients at 1 and 3 h and quantified the SUV and retention index SUV (RI-SUV) for comparison with CT findings. A monthly pulmonary function test was done after FDG PET/CT study to assess disease progression. Early cryptogenic organizing pneumonia (COP) had higher SUVs as compared to idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP). They suggested that the early SUV value might be used as a marker for differentiation of COP from NSIP and IPF. It was also shown that a positive RI-SUV predicts deterioration of lung function in IIP patients. Early SUV and RI-SUV parameters evaluated with DTPI might predict disease progression and treatment response to steroids in IIP patients soon after medical evaluation.
Pulmonary sarcoidosis
Dual time point FDG PET/CT imaging has also been used for the prediction of disease progression in pulmonary sarcoidosis. Umeda et al. [46] scanned 21 patients with pulmonary sarcoidosis at 1 and 3 h post injection. SUVs and RI-SUVs were calculated and disease progression was evaluated on the basis of a chest CT performed a year after FDG PET/CT. RI-SUVs were significantly higher in patients with increased or unchanged lesions on follow-up CT when compared with patients with lesions showing a lower retention index (RI). RI-SUVs showed greater diagnostic accuracy when compared with the use of early conventional single time point imaging (STPI) SUV measurement and serum soluble IL-2 and 67Ga uptake in the group examined. They concluded that RI-SUVs might be used for measurement of persistent inflammation in patients with pulmonary sarcoidosis.
Crohn’s disease
It has been shown that DTPI might also be able to predict potential response to treatment with antitumor necrosis factor (TNF) drugs in patients with Crohn’s disease (CD) [47]. In this preliminary study, nine patients with CD were evaluated using DTPI before and after treatment and response to treatment was quantified as the difference between pre-treatment and post-treatment global CD activity and FDG RI between the first and the second hour after the administration of the radiotracer. Treatment response was shown to be correlated with pre-treatment RI with a correlation coefficient of 0.76 (p = 0.01), suggesting that pre-treatment RI can be an important predictor of response to anti-TNF therapy. Further studies with larger study samples are needed to define the role of DTPI in CD.
Brown fat
Brown adipose tissue is a known source of false-positive results in FDG PET studies [48, 49]. Brown fat tissues are visualized as bilaterally elongated and symmetrical structures in the supra clavicular area and are infrequently interpreted as malignant lesions or nodal metastases [50, 51]. Alkhawaldeh et al. [48], implementing DTPI, quantitatively assessed FDG PET scans from 32 patients for hypermetabolic brown fat activity and noted diverse patterns of distribution of brown fat throughout the body including the supraclavicular, cervical, axillary, paravertebral, mediastinal, upper abdominal and intercostal regions. The SUVmax ranged from 0.8 to 12.4 at these uptake sites over time. 76 % of the brown fat sites showed increased uptake which ranged from 12 to 192 %, while 13 % did not change and 11 % showed decreased values.
Malignant diseases
Lung cancer
Diagnostic performance
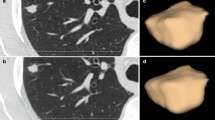
Two-thirds of pulmonary nodules are benign (mostly due to inflammatory reactions) and the rest are malignant in nature [52, 53]. It is now well established that FDG PET is beneficial in the diagnosis and staging of lung cancer lesions [54] (Fig. 3). However, false-positive [9] and false-negative [24] results have been reported in the literature.
The images above (a, b, c) demonstrate the importance of delayed imaging in assessing the degree of aggressiveness of the primacy malignant lesion but also in improving the sensitivity of the technique for detecting regional and distant metastatic sites. In this patient with lung cancer, the primary lesion was found to show a substantial rise in the degree of FDG uptake, both qualitative and quantitative, over time. In addition, pleural involvement on the same side was undetectable in the images acquired at 1 h after administration of FDG but became visible over time and appeared very intense at 3 h (arrows). The table (d) further confirms this observation by providing quantitative values for both the primary lesion and the involved pleura. The table provides conventional and novel quantitative measurements (MTV metabolic tumor volume, TLG total lesion glycolysis, SUVmax, maximum standardized uptake value) (color figure online)
Matthies et al. [24] reported sensitivity and specificity values of 80 and 94 %, respectively, for a cutoff SUV of 2.5 on the standard FDG PET scan. In their study, DTPI was found to increase the sensitivity to 100 %, but did not significantly change the specificity of the test (89 %).
Alkhawaldeh et al. [55] in another study using DTPI, found it to improve the diagnostic accuracy of FDG PET in the assessment of solitary pulmonary nodules.
Cheng et al. [21] prospectively assessed dynamic changes in FDG uptake in patients with proven or suspected lung cancer at 1, 2, and 3 h post-injection and concluded that multiple time point imaging moderately improves the diagnostic accuracy of FDG PET in assessing lung lesions. The SUVmax of 4.21 at the third hour was found to show the best diagnostic performance (=88 %). The TBR increased over time and the overall quality of the images on the delayed images appeared to be superior to that of the early scans.
Lin et al. [56], in their systematic review and meta-analysis of 11 studies comprising 788 patients, conducted to assess the potential value of dual time point versus single time point FDG PET imaging, found the area under curve for DTPI and STPI to be 0.839 (0.079) and 0.757 (0.074), respectively. Their analysis demonstrated that DTPI may not be recommended for routine clinical use. However, it may provide additional information in specific non-diagnostic settings where STPI is of limited value in characterizing lesions.
Zhang et al. [57] in their meta-analysis of eight studies (for a total of 415 patients and 430 pulmonary nodules), reported a sensitivity of 79 % (95 % CI 74.0–84.0 %) and a specificity of 73 % (95 % CI 65–79) for DTPI. STPI had a sensitivity of 77 % (95 % CI 71.9–82.3 %) and a specificity of 59 % (95 % CI 0.29–0.49). They concluded that DTPI and STPI with FDG PET show relatively similar accuracy for differentiating pulmonary nodules. However, DTPI appeared, on the basis of this meta-analysis, to be more specific than STPI.
Prognostic performance
FDG uptake parameters generated from either DTPI or delayed time-point imaging alone can be used for predicting the outcome of lung malignancies. Houseni et al. [58], in their study, reported SUVmax changes from 1 to 1.5 h as a strong independent predictive factor for lung cancer prognosis. On the basis of these data a more than 25 % increase in the SUVmax predicted significantly shorter overall survival time as compared to that recorded in the group with values that were <25 %. Chen et al. [59] studied the prognostic value of DTPI in patients with nonsmall cell lung cancer by measuring the increment in SUVmax (SUVinc) between the first and second hour. They noted that the cutoff value of >1 for SUVinc over time had the best prognostic value for progression-free survival. The 3-year progression-free survival and overall survival values were 61.6 and 87.8 % in patients with SUVinc ≤ 1 versus 21.1 and 46.2 % in patients with SUVinc > 1 (all p < 0.01). The authors concluded that DTPI provides a promising prognostic value for determining the outcome in nonsmall cell lung cancer.
In contrast, Kim et al. [60] reported that the percentage change in SUVmax recorded in DTPI may not predict outcome. The %∆ SUVmax did not predict overall survival or disease-free survival. However, tumor SUVmax in early images was a significant predictive factor for overall survival (p = 0.0142) and disease-free survival (p = 0.0421) in surgically resected early stage non-small cell lung cancer.
Satoh et al. [61] showed that SUVmax is not able to predict recurrence or survival times. In contrast, the RI could detect and determine the number of distant metastatic lesions and, therefore, predict local recurrence rates and regional lymph node metastasis.
Lymph node staging of lung cancer
Dual time point imaging (DTPI) has been studied as a means of detecting of lymph node metastasis in lung cancer. Accurate detection of lymph node metastasis is crucial for treatment planning. Shen et al. [62], in their meta-analysis, evaluated the diagnostic performance of DTPI and STPI with FDG PET for the detection of mediastinal nodal metastasis in non small cell lung cancer. DTPI with FDG PET performed slightly better than STPI with FDG PET in the evaluation of mediastinal lymph nodes. However, due to the small sample of patients and the heterogeneity of the population examined, future studies should be carried out to determine what role DTPI might play for this purpose.
Shinozaki et al. [63] examined the diagnostic accuracy of DTPI versus STPI with FDG PET in the pre-operative staging of lung cancer in 100 patients. Early time point imaging with FDG PET resulted in upstaging of the tumor in 10 % and down staging of the tumor in 5 % of the cases. However, DTPI did not appear to add any additional information to the overall staging of the lung cancer patients. This finding suggests that although DTPI is useful for differentiating between malignant and benign lesions, overall it has no major impact on the staging and management of patients with lung cancer.
Breast
FDG PET has been used for diagnosing and staging breast cancer and for detecting recurrence of the disease. Most breast cancers are low-grade malignancies and small in size in many patients and these are two of the factors that limit the applicability of FDG PET in this malignancy [44]. Mavi et al. [11, 15] studied DTPI in a relatively large number of breast cancer patients. Their study included 152 patients scanned twice with a mean interval of 52 min between the two images. They observed an increase in FDG uptake over time in malignant lesions when compared with normal breast tissue. They also noted that changes in FDG uptake at different time points may reflect the tumor biology and the degree of aggressiveness of the malignant lesion.
Caprio et al. [28] assessed the diagnostic performance of DTPI in suspected breast cancer lesions. They studied 59 patients at 1 and 3 h after FDG injection, qualitatively and semiquantitatively evaluating the changes in FDG uptake parameters and comparing them with the results from histopathological examinations of the excised lesions. DTPI showed an accuracy of 85 % for the lesions with SUVmax above or equal to 2.5 and/or positive percent change in SUVmax. This parameter had a sensitivity of 81 % and a specificity of 100 %, when compared with accuracy, sensitivity and specificity values of 69, 63, and 100 % in STPI, respectively. They concluded that DTPI, when compared with STPI alone, improves the breast cancer detection accuracy in patients with suspicious lesions.
Head and neck
Generally there is a considerable degree of physiological FDG uptake in the head and neck region. Furthermore, episodes of inflammation and infections in the upper respiratory tract result in increased FDG uptake in the affected sites. Radiation-induced inflammation is also a leading cause of false-positive FDG uptake in head and neck cancers. DTPI has been employed in this setting to determine its role in evaluating the complex anatomical structures of this region and the preliminary results, with regard to the differentiation of benign and malignant lesions, appear to be promising [44].
Hustinx et al. [8] used DTPI for the assessment of head and neck lesions and noted that while SUV levels were similar in tumors and inflammation on baseline scans, over time, FDG uptake in the tumors increased by 30 % whereas uptake in inflammatory or normal tissues remained stable.
Abgral et al. [29] prospectively investigated the independent prognostic value of DTPI with FDG PET in patients with head and neck squamous cell carcinoma at 1 and 2-h image acquisition. The intra-tumoral RI was measured. Event-free survival and overall survival were compared with SUVmax at different time points. Age, stage and RI were predictive of event-free survival (p = 0.01) only. SUVmax at 1 h was not predictive of event-free survival or overall survival. At the second time point, SUVmax was predictive of overall survival, but not event-free survival. On multivariate analysis, the RI emerged as the only predictive factor for event-free survival.
Limitations
Dual time point imaging (DTPI) has certain limitations, which makes its utility in routine clinical practice somewhat unclear at this time [2]. The use of DTPI to differentiate between inflammatory and malignant lesions has not been consistently successful in every setting, as discussed in this review. A number of studies have shown a significant non-specificity of this approach for differentiating between benign and malignant lesions, especially, in the lung and mediastinal regions [13, 46, 64–68] and in lung nodules with low FDG avidity [69]. FDG uptake in acute inflammatory lesions, particularly those related to granulomatous/infectious lesions, mimics a pattern seen in malignant lesions [70, 71]. For example, DTPI, when used in tuberculosis-endemic regions or areas with a high prevalence of sarcoidosis, has not shown additional value over STPI [13, 46, 64–68, 72]. Conversely, in chronic inflammatory (and infectious) foci whose FDG uptake shows a decline after a certain time point, metabolically active cells appear to retain FDG-6-phosphate in a manner similar to that of malignant cells. The discrepancy in the results between acute and chronic inflammatory lesions is likely related to the biological behavior of inflammatory cells in these two different settings [2]. Therefore, it is our belief that DTPI can be used for differentiation of malignant from chronic inflammatory sites. Brown fat tends to accumulate FDG over time and can be considered as a confounding factor in DTPI [48]. Some concerns have been raised about DTPI-based evaluation of suspicious focal abdominal FDG uptake, likely due to methodological problems in the studies [44, 73].
Summary
DTPI methodology has been shown to provide useful diagnostic and prognostic information in certain situations, which may improve the sensitivity, specificity and accuracy of FDG PET studies. Although further, large-scale multicenter studies are required to determine the definitive value of DTPI for use on a routine basis, this approach can already be used in specific settings and conditions in which promising results have been reported.
References
Hess S, Blomberg BA, Zhu HJ, Hoilund-Carlsen PF, Alavi A (2014) The pivotal role of FDG-PET/CT in modern medicine. Acad Radiol 21:232–249. doi:10.1016/j.acra.2013.11.002
Cheng G, Torigian DA, Zhuang H, Alavi A (2013) When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging 40:779–787. doi:10.1007/s00259-013-2343-9
Shinya T, Fujii S, Asakura S, Taniguchi T, Yoshio K, Alafate A, Sato S, Yoshino T, Kanazawa S (2012) Dual-time-point F-18 FDG PET/CT for evaluation in patients with malignant lymphoma. Ann Nucl Med 26:616–621. doi:10.1007/s12149-012-0619-y
Lee JW, Kim SK, Lee SM, Moon SH, Kim TS (2011) Detection of hepatic metastases using dual-time-point FDG PET/CT scans in patients with colorectal cancer. Mol Imaging Biol 13:565–572. doi:10.1007/s11307-010-0394-x
Schillaci O, Travascio L, Bolacchi F, Calabria F, Bruni C, Ciccio C, Guazzaroni M, Orlacchio A, Simonetti G (2009) Accuracy of early and delayed FDG PET-CT and of contrast-enhanced CT in the evaluation of lung nodules: a preliminary study on 30 patients. Radiol Med 114:890–906. doi:10.1007/s11547-009-0400-z
Xiu Y, Bhutani C, Dhurairaj T, Yu JQ, Dadparvar S, Reddy S, Kumar R, Yang H, Alavi A, Zhuang H (2007) Dual-time point FDG PET imaging in the evaluation of pulmonary nodules with minimally increased metabolic activity. Clin Nucl Med 32:101–105. doi:10.1097/01.rlu.0000252457.54929.b7
Kumar R, Loving VA, Chauhan A, Zhuang H, Mitchell S, Alavi A (2005) Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med 46:1819–1824
Hustinx R, Smith RJ, Benard F, Rosenthal DI, Machtay M, Farber LA, Alavi A (1999) Dual time point fluorine-18 fluorodeoxyglucose positron emission tomography: a potential method to differentiate malignancy from inflammation and normal tissue in the head and neck. Eur J Nucl Med 26:1345–1348
Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, Lanuti M, Li P, Mozley PD, Rossman MD, Albelda SM, Alavi A (2001) Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med 42:1412–1417
Sahlmann CO, Siefker U, Lehmann K, Meller J (2004) Dual time point 2-[18F]fluoro-2’-deoxyglucose positron emission tomography in chronic bacterial osteomyelitis. Nucl Med Commun 25:819–823
Mavi A, Urhan M, Yu JQ, Zhuang H, Houseni M, Cermik TF, Thiruvenkatasamy D, Czerniecki B, Schnall M, Alavi A (2006) Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med 47:1440–1446
Basu S, Alavi A (2007) Partial volume correction of standardized uptake values and the dual time point in FDG-PET imaging: should these be routinely employed in assessing patients with cancer? Eur J Nucl Med Mol Imaging 34:1527–1529. doi:10.1007/s00259-007-0467-5
Yen RF, Chen KC, Lee JM, Chang YC, Wang J, Cheng MF, Wu YW, Lee YC (2008) 18F-FDG PET for the lymph node staging of non-small cell lung cancer in a tuberculosis-endemic country: is dual time point imaging worth the effort? Europ J Nuclear Med Mol Imaging 35:1305–1315. doi:10.1007/s00259-008-0733-1
Alkhawaldeh K, Biersack HJ, Henke A, Ezziddin S (2011) Impact of dual-time-point F-18 FDG PET/CT in the assessment of pleural effusion in patients with non-small-cell lung cancer. Clin Nucl Med 36:423–428. doi:10.1097/RLU.0b013e3182173823
Basu S, Kwee TC, Surti S, Akin EA, Yoo D, Alavi A (2011) Fundamentals of PET and PET/CT imaging. Ann N Y Acad Sci 1228:1–18. doi:10.1111/j.1749-6632.2011.06077.x
Nelson CA, Wang JQ, Leav I, Crane PD (1996) The interaction among glucose transport, hexokinase, and glucose-6-phosphatase with respect to 3H-2-deoxyglucose retention in murine tumor models. Nucl Med Biol 23:533–541
Suzuki S, Toyota T, Suzuki H, Goto Y (1984) Partial purification from human mononuclear cells and placental plasma membranes of an insulin mediator which stimulates pyruvate dehydrogenase and suppresses glucose-6-phosphatase. Arch Biochem Biophys 235:418–426
Higashi T, Saga T, Nakamoto Y, Ishimori T, Mamede MH, Wada M, Doi R, Hosotani R, Imamura R, Konishi J (2002) Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med 43:173–180
Chang CC, Cho SF, Chen YW, Tu HP, Lin CY, Chang CS (2012) SUV on dual-phase FDG PET/CT correlates with the Ki-67 proliferation index in patients with newly diagnosed non-Hodgkin lymphoma. Clin Nucl Med 37:e189–e195. doi:10.1097/RLU.0b013e318251e16e
Barger RL Jr, Nandalur KR (2012) Diagnostic performance of dual-time 18F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol 19:153–158. doi:10.1016/j.acra.2011.10.009
Cheng G, Alavi A, Werner TJ, Del Bello CV, Akers SR (2014) Serial changes of FDG uptake and diagnosis of suspected lung malignancy: a lesion-based analysis. Clin Nucl Med 39:147–155. doi:10.1097/RLU.0000000000000313
Kaneko K, Sadashima E, Irie K, Hayashi A, Masunari S, Yoshida T, Omagari J (2013) Assessment of FDG retention differences between the FDG-avid benign pulmonary lesion and primary lung cancer using dual-time-point FDG-PET imaging. Ann Nucl Med 27:392–399. doi:10.1007/s12149-013-0698-4
Khan AN, Al-Jahdali H (2013) Value of delayed 18F-FDG PET in the diagnosis of solitary pulmonary nodule. J Thorac Dis 5:373–374. doi:10.3978/j.issn.2072-1439.2013.06.05
Matthies A, Hickeson M, Cuchiara A, Alavi A (2002) Dual time point 18F-FDG PET for the evaluation of pulmonary nodules. J Nucl Med 43:871–875
Garcia Vicente AM, Soriano Castrejon A, Cruz Mora MA, Ortega Ruiperez C, Espinosa Aunion R, Leon Martin A, Gonzalez Ageitos A, Van Gomez LO (2014) Dual time point 2-deoxy-2-[18F]fluoro-d-glucose PET/CT: nodal staging in locally advanced breast cancer. Rev Esp Med Nucl Imagen Mol 33:1–5. doi:10.1016/j.remn.2013.03.005
Garcia Vicente AM, Soriano Castrejon A, Relea Calatayud F, Munoz Madero V, Molina Garrido MJ, Leon Martin AA, Cordero Garcia JM, Pilkington Woll JP, Chacon Lopez-Muniz I, Palomar Munoz A (2012) 18F-FDG semi-quantitative parameters and biological prognostic factors in locally advanced breast cancer. Rev Esp Med Nucl Imagen Mol 31:308–314. doi:10.1016/j.remn.2011.12.001
Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E (2008) Dual time point FDG-PET/CT imaging. Potential tool for diagnosis of breast cancer. Clin Radiol 63:1213–1227. doi:10.1016/j.crad.2008.03.014
Caprio MG, Cangiano A, Imbriaco M, Soscia F, Di Martino G, Farina A, Avitabile G, Pace L, Forestieri P, Salvatore M (2010) Dual-time-point [18F]-FDG PET/CT in the diagnostic evaluation of suspicious breast lesions. Radiol Med 115:215–224. doi:10.1007/s11547-009-0491-6
Abgral R, Le Roux PY, Rousset J, Querellou S, Valette G, Nowak E, Turzo A, Tissot V, Marianowski R, Salaun PY (2013) Prognostic value of dual-time-point 18F-FDG PET-CT imaging in patients with head and neck squamous cell carcinoma. Nucl Med Commun 34:551–556. doi:10.1097/MNM.0b013e32836089ab
Lee JH, Lee WA, Park SG, Park DK, Namgung H (2012) Relationship between dual-time point FDG PET and immunohistochemical parameters in preoperative colorectal cancer: preliminary study. Nucl Med Mol Imaging 46:48–56. doi:10.1007/s13139-011-0120-x
Yoon HJ, Kim SK, Kim TS, Im HJ, Lee ES, Kim HC, Park JW, Chang HJ, Choi HS, Kim DY, Oh JH (2013) New application of dual point 18F-FDG PET/CT in the evaluation of neoadjuvant chemoradiation response of locally advanced rectal cancer. Clin Nucl Med 38:7–12. doi:10.1097/RLU.0b013e3182639a58
Kim DW, Jung SA, Kim CG, Park SA (2010) The efficacy of dual time point F-18 FDG PET imaging for grading of brain tumors. Clin Nucl Med 35:400–403. doi:10.1097/RLU.0b013e3181db4cfb
Prieto E, Marti-Climent JM, Dominguez-Prado I, Garrastachu P, Diez-Valle R, Tejada S, Aristu JJ, Penuelas I, Arbizu J (2011) Voxel-based analysis of dual-time-point 18F-FDG PET images for brain tumor identification and delineation. J Nucl Med 52:865–872. doi:10.2967/jnumed.110.085324
Nakayama M, Okizaki A, Ishitoya S, Sakaguchi M, Sato J, Aburano T (2013) Dual-time-point F-18 FDG PET/CT imaging for differentiating the lymph nodes between malignant lymphoma and benign lesions. Ann Nucl Med 27:163–169. doi:10.1007/s12149-012-0669-1
Costantini DL, Vali R, Chan J, McQuattie S, Charron M (2013) Dual-time-point FDG PET/CT for the evaluation of pediatric tumors. AJR Am J Roentgenol 200:408–413. doi:10.2214/ajr.12.8930
Nishiyama Y, Yamamoto Y, Fukunaga K, Kimura N, Miki A, Sasakawa Y, Wakabayashi H, Satoh K, Ohkawa M (2006) Dual-time-point 18F-FDG PET for the evaluation of gallbladder carcinoma. J Nucl Med 47:633–638
Blomberg BA, Akers SR, Saboury B, Mehta NN, Cheng G, Torigian DA, Lim E, Del Bello C, Werner TJ, Alavi A (2013) Delayed time-point 18F-FDG PET CT imaging enhances assessment of atherosclerotic plaque inflammation. Nucl Med Commun 34:860–867. doi:10.1097/MNM.0b013e3283637512
Blomberg BA, Thomassen A, Takx RA, Hildebrandt MG, Simonsen JA, Buch-Olsen KM, Diederichsen AC, Mickley H, Alavi A, Hoilund-Carlsen PF (2014) Delayed 18F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol 21:588–597. doi:10.1007/s12350-014-9884-6
Cheng G, Alavi A, Del Bello CV, Akers SR (2013) Differential washout of FDG activity in two different inflammatory lesions: implications for delayed imaging. Clin Nucl Med 38:576–579. doi:10.1097/RLU.0b013e318292efc8
Cheng G, Alavi A, Lee NJ, Akers SR (2014) Differential background clearance of fluorodeoxyglucose activity in normal tissues and its clinical significance. PET Clin 9:209–216. doi:10.1016/j.cpet.2013.12.001
Cheng G, Alavi A, Lim E, Werner TJ, Del Bello CV, Akers SR (2013) Dynamic changes of FDG uptake and clearance in normal tissues. Mol Imaging Biol 15:345–352. doi:10.1007/s11307-012-0600-0
Basu S, Zaidi H, Houseni M, Bural G, Udupa J, Acton P, Torigian DA, Alavi A (2007) Novel quantitative techniques for assessing regional and global function and structure based on modern imaging modalities: implications for normal variation, aging and diseased states. Semin Nucl Med 37:223–239. doi:10.1053/j.semnuclmed.2007.01.005
Basu S, Kung J, Houseni M, Zhuang H, Tidmarsh GF, Alavi A (2009) Temporal profile of fluorodeoxyglucose uptake in malignant lesions and normal organs over extended time periods in patients with lung carcinoma: implications for its utilization in assessing malignant lesions. Q J Nucl Med Mol Imaging 53:9–19
Sanz-Viedma S, Torigian DA, Parsons M, Basu S, Alavi A (2009) Potential clinical utility of dual time point FDG-PET for distinguishing benign from malignant lesions: implications for oncological imaging. Rev Esp Med Nucl 28:159–166
Umeda Y, Demura Y, Ishizaki T, Ameshima S, Miyamori I, Saito Y, Tsuchida T, Fujibayashi Y, Okazawa H (2009) Dual-time-point 18F-FDG PET imaging for diagnosis of disease type and disease activity in patients with idiopathic interstitial pneumonia. Euro J Nuclear Med Mol Imaging 36:1121–1130. doi:10.1007/s00259-009-1069-1
Umeda Y, Demura Y, Morikawa M, Ameshima S, Tsuchida T, Fujibayashi Y, Okazawa H, Ishizaki T (2011) Prognostic value of dual-time-point 18F-fluorodeoxyglucose positron emission tomography in patients with pulmonary sarcoidosis. Respirology 16:713–720. doi:10.1111/j.1440-1843.2011.01966.x
Saboury B, Salavati A, Sanz-Viedma S, Alavi A (2013) Pre-treatment dual-time-point FDG-PET/CT imaging may predict response in patients with Crohn’s disease. J Nucl Med, Meeting Abstracts 54 (2_MeetingAbstracts):587
Alkhawaldeh K, Alavi A (2008) Quantitative assessment of FDG uptake in brown fat using standardized uptake value and dual-time-point scanning. Clin Nucl Med 33:663–667. doi:10.1097/RLU.0b013e318184b3de
Basu S, Alavi A (2008) Optimizing interventions for preventing uptake in the brown adipose tissue in FDG-PET. Eur J Nucl Med Mol Imaging 35:1421–1423. doi:10.1007/s00259-008-0720-6
Barrington SF, Maisey MN (1996) Skeletal muscle uptake of fluorine-18-FDG: effect of oral diazepam. J Nucl Med 37:1127–1129
Gordon BA, Flanagan FL, Dehdashti F (1997) Whole-body positron emission tomography: normal variations, pitfalls, and technical considerations. AJR Am J Roentgenol 169:1675–1680. doi:10.2214/ajr.169.6.9393189
Higgins GA, Shields TW, Keehn RJ (1975) The solitary pulmonary nodule. Ten-year follow-up of veterans administration-armed forces cooperative study. Arch Surg 110:570–575
Lillington GA, Caskey CI (1993) Evaluation and management of solitary and multiple pulmonary nodules. Clin Chest Med 14:111–119
Laffon E, de Clermont H, Begueret H, Vernejoux JM, Thumerel M, Marthan R, Ducassou D (2009) Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun 30:455–461. doi:10.1097/MNM.0b013e32832bdcac
Alkhawaldeh K, Bural G, Kumar R, Alavi A (2008) Impact of dual-time-point 18F-FDG PET imaging and partial volume correction in the assessment of solitary pulmonary nodules. Eur J Nuclear Med Mol Imaging 35:246–252. doi:10.1007/s00259-007-0584-1
Lin YY, Chen JH, Ding HJ, Liang JA, Yeh JJ, Kao CH (2012) Potential value of dual-time-point 18F-FDG PET compared with initial single-time-point imaging in differentiating malignant from benign pulmonary nodules: a systematic review and meta-analysis. Nucl Med Commun 33:1011–1018. doi:10.1097/MNM.0b013e32835710d6
Zhang L, Wang Y, Lei J, Tian J, Zhai Y (2013) Dual time point 18FDG-PET/CT versus single time point 18FDG-PET/CT for the differential diagnosis of pulmonary nodules: a meta-analysis. Acta Radiol 54:770–777. doi:10.1177/0284185113481594
Houseni M, Chamroonrat W, Zhuang J, Gopal R, Alavi A, Zhuang H (2010) Prognostic implication of dual-phase PET in adenocarcinoma of the lung. J Nucl Med 51:535–542. doi:10.2967/jnumed.109.068643
Chen HH, Lee BF, Su WC, Lai YH, Chen HY, Guo HR, Yao WJ, Chiu NT (2013) The increment in standardized uptake value determined using dual-phase 18F-FDG PET is a promising prognostic factor in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging 40:1478–1485. doi:10.1007/s00259-013-2452-5
Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK (2011) Limited prognostic value of dual time point F-18 FDG PET/CT in patients with early stage (stage I & II) non-small cell lung cancer (NSCLC). Radiother Oncol 98:105–108. doi:10.1016/j.radonc.2010.11.007
Satoh Y, Nambu A, Onishi H, Sawada E, Tominaga L, Kuriyama K, Komiyama T, Marino K, Aoki S, Araya M, Saito R, Maehata Y, Oguri M, Araki T (2012) Value of dual time point F-18 FDG-PET/CT imaging for the evaluation of prognosis and risk factors for recurrence in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Eur J Radiol 81:3530–3534. doi:10.1016/j.ejrad.2011.11.047
Shen G, Hu S, Deng H, Jia Z (2014) Diagnostic value of dual time-point 18 F-FDG PET/CT versus single time-point imaging for detection of mediastinal nodal metastasis in non-small cell lung cancer patients: a meta-analysis. Acta Radiol. doi:10.1177/0284185114535210
Shinozaki T, Utano K, Fujii H, Utano Y, Sasaki T, Kijima S, Kanazawa H, Kimura Y, Fujita A, Sugimoto H (2014) Routine use of dual time 18F-FDG PET for staging of preoperative lung cancer: does it affect clinical management? Jpn J Radiol. doi:10.1007/s11604-014-0336-7
Li M, Wu N, Liu Y, Zheng R, Liang Y, Zhang W, Zhao P (2012) Regional nodal staging with 18F-FDG PET-CT in non-small cell lung cancer: additional diagnostic value of CT attenuation and dual-time-point imaging. Eur J Radiol 81:1886–1890. doi:10.1016/j.ejrad.2011.03.074
Chen C-J, Lee B-F, Yao W-J, Cheng L, Wu P-S, Chu CL, Chiu N-T (2008) Dual-phase 18F-FDG PET in the diagnosis of pulmonary nodules with an initial standard uptake value less than 2.5. AJR Am J Roentgenol 191:475–479
Laffon E, de Clermont H, Begueret H, Vernejoux J-M, Thumerel M, Marthan R, Ducassou D (2009) Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun 30:455–461
Sathekge MM, Maes A, Pottel H, Stoltz A, van de Wiele C (2010) Dual time-point FDG PET-CT for differentiating benign from malignant solitary pulmonary nodules in a TB endemic area. S Afr Med J 100:598–601
Zheng Z, Pan Y, Guo F, Wei H, Wu S, Pan T, Li J (2011) Multimodality FDG PET/CT appearance of pulmonary tuberculoma mimicking lung cancer and pathologic correlation in a tuberculosis-endemic country. South Med J 104:440–445
Cloran FJ, Banks KP, Song WS, Kim Y, Bradley YC (2010) Limitations of dual time point PET in the assessment of lung nodules with low FDG avidity. Lung Cancer 68:66–71. doi:10.1016/j.lungcan.2009.05.013
Kok PJ, van Eerd JE, Boerman OC, Corstens FH, Oyen WJ (2005) Biodistribution and imaging of FDG in rats with LS174T carcinoma xenografts and focal Escherichia coli infection. Cancer Biother Radiopharm 20:310–315. doi:10.1089/cbr.2005.20.310
Makinen TJ, Lankinen P, Poyhonen T, Jalava J, Aro HT, Roivainen A (2005) Comparison of 18F-FDG and 68Ga PET imaging in the assessment of experimental osteomyelitis due to Staphylococcus aureus. Eur J Nucl Med Mol Imaging 32:1259–1268. doi:10.1007/s00259-005-1841-9
Kim D-W, Kim CG (2011) Dual-time point positron emission tomography findings of benign mediastinal lymph nodes in a tuberculosis-endemic region. Jpn J Radiol 29:682–687
Dobert N, Hamscho N, Menzel C, Neuss L, Kovacs AF, Grunwald F (2004) Limitations of dual time point FDG-PET imaging in the evaluation of focal abdominal lesions. Nuklearmedizin 43:143–149. doi:10.1267/NUKL04050143
Lee S, Park T, Park S, Pahk K, Rhee S, Cho J, Jeong E, Kim S, Choe JG (2014) The clinical role of dual-time-point 18F-FDG PET/CT in differential diagnosis of the thyroid incidentaloma. Nucl Med Mol Imaging 48(2):121–129. doi:10.1007/s13139-013-0247-z
Kim DW, Kim WH, Kim CG (2012) Dual-time-point FDG PET/CT: Is it useful for lymph node staging in patients with non-small-cell lung cancer? Nucl Med Mol Imaging 46:196–200. doi:10.1007/s13139-012-0141-0
Kim SJ, Kim YK, Kim IJ, Kim YD, Lee MK (2011) Limited predictive value of dual-time-point F-18 FDG PET/CT for evaluation of pathologic N1 status in NSCLC patients. Clin Nucl Med 36:434–439. doi:10.1097/RLU.0b013e31820adef8
Macdonald K, Searle J, Lyburn I (2011) The role of dual time point FDG PET imaging in the evaluation of solitary pulmonary nodules with an initial standard uptake value less than 2.5. Clin Radiol 66:244–250. doi:10.1016/j.crad.2010.10.008
Toriihara A, Nakamura S, Kubota K, Makino T, Okochi K, Shibuya H (2013) Can dual-time-point 18F-FDG PET/CT differentiate malignant salivary gland tumors from benign tumors? AJR Am J Roentgenol 201:639–644. doi:10.2214/AJR.12.10395
Matthiessen LW, Johannesen HH, Skougaard K, Gehl J, Hendel HW (2013) Dual time point imaging fluorine-18 flourodeoxyglucose positron emission tomography for evaluation of large loco-regional recurrences of breast cancer treated with electrochemotherapy. Radiol Oncol 47:358–365. doi:10.2478/raon-2013-0054
Choi EK, Yoo Ie R, Kim SH, O JH, Choi WH, Na SJ, Park SY (2013) The clinical value of dual-time point 18F-FDG PET/CT for differentiating extrahepatic cholangiocarcinoma from benign disease. Clin Nucl Med 38:e106–e111. doi:10.1097/RLU.0b013e318266f402
Shum WY, Hsieh TC, Yeh JJ, Chen JH, Su CC, Liang JA, Kao CH (2012) Clinical usefulness of dual-time FDG PET-CT in assessment of esophageal squamous cell carcinoma. Eur J Radiol 81:1024–1028. doi:10.1016/j.ejrad.2011.03.018
Miyake KK, Nakamoto Y, Togashi K (2012) Dual-time-point 18F-FDG PET/CT in patients with colorectal cancer: clinical value of early delayed scanning. Ann Nucl Med 26:492–500. doi:10.1007/s12149-012-0599-y
Hahn S, Hecktor J, Grabellus F, Hartung V, Poppel T, Kimmig R, Forsting M, Antoch G, Heusner TA (2012) Diagnostic accuracy of dual-time-point 18F-FDG PET/CT for the detection of axillary lymph node metastases in breast cancer patients. Acta Radiol 53:518–523. doi:10.1258/ar.2012.110420
Kim SJ, Kim BH, Jeon YK, Kim SS, Kim IJ (2011) Limited diagnostic and predictive values of dual-time-point 18F FDG PET/CT for differentiation of incidentally detected thyroid nodules. Ann Nucl Med 25:347–353. doi:10.1007/s12149-011-0468-0
Conflict of interest
The authors of this review article (Sina Houshmand, Ali Salavati, Sandip Basu, Benjapa Khiewvan, Abass Alavi) declare that they have no conflict of interest.
Human and Animal Studies
The manuscript does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
S. Houshmand and A. Salavati contributed equally to this study.
Color figures online at http://link.springer.com/article/10.1007/s40336-014-0075-x
Rights and permissions
About this article
Cite this article
Houshmand, S., Salavati, A., Basu, S. et al. The role of dual and multiple time point imaging of FDG uptake in both normal and disease states. Clin Transl Imaging 2, 281–293 (2014). https://doi.org/10.1007/s40336-014-0075-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-014-0075-x