Abstract
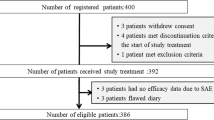
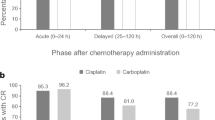
Lung cancer is the leading cause of cancer-related death for both men and women worldwide, and lung cancer also has the highest morbidity and mortality rate among all cancers in China. Chemotherapy (CT) is the most effective and most widely used treatment for lung cancer. Nausea and vomiting are still among the most unpleasant side effects of chemotherapy, especially during highly emetogenic chemotherapy. The standard therapy for preventing chemotherapy-induced nausea and vomiting (CINV) is 5-hydroxytryptamine 3 (5-HT3) receptor antagonists. Palonosetron is a highly potent second-generation selective 5-HT3 receptor antagonist with stronger binding affinity for the 5-HT3 receptor. Palonosetron showed a high antiemetic activity in preclinical study and pivotal trails enrolling patients treated with moderately or high antiemetic activity drugs. Aim of the study was to verify the activity and safety of palonosetron in patients affected by non-small-cell lung carcinoma (NSCLC) and treated with chemotherapy. Patients with stage II–IV NSCLC and receiving chemotherapy entered into the trial. Informed written consent was required. Patients were randomized to received palonosetron or ondasetron. A single pretreatment dose of palonosetron 0.25 mg intravenous followed was administered. Nausea and vomiting were evaluated over 7-day period. Also the adverse effects were reported. Adverse events were evaluated according to the NCI-CTC criteria. Eighty-nine patients were enrolled into the study. The complete responses during the acute phase were 95.4 and 93.3%, respectively. The main side effects were headache 4.5%, constipation 15.7%, anxiety 2.3%. Palonosetron is a very active antiemetic drug for the prevention of nausea and vomiting in NSCLC patients received chemotherapy.
Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Navari RM, Einhorn LH, Loehrer PJ Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer. 2007;15:1285–91.
Grunberg SM, Deuson RR, Mavros P, et al. Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer. 2004;100:2261–8.
Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472–8.
Hesketh PJ. Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Invest. 2000;18:163–73.
Kris M, Gralla R, Clark R, et al. Incidence, course and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3:1379–84.
Walton SM. Advances in use of the 5-HT3 receptor antagonists. Exp Opin Pharmacother. 2000;1:207–23.
Gebbia V, Cannata G, Testa A, et al. Ondansetron versus granisetron in the prevention of chemotherapy-induced nausea and vomiting. Cancer. 1994;74:1945–52.
Perez EA, Hesketh P, Sandbach J, et al. Comparison of single-dose oral granisetron versus intravenous ondansetron in the prevention of nausea and vomiting induced moderately emetogenic chemotherapy: a multicenter, double-blind, randomized parallel study. J Clin Oncol. 1998;16:754–60.
Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: up date 2006. J Clin Oncol. 2006;24:2932–47.
Gralla R, Lichinitser S, Van Der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–7.
Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist Results of a phase III single-dose trial versus dolasetron. Cancer. 2003;98(11):2473–82.
Aapro M, Grunberg S, Manikhas G, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2004;17:1441–9.
National Cancer Institute-Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006 [August9].
Einhorn LH, Rapoport B, Koeller J, et al. Antiemetic therapy for multiple-day chemotherapy and high-dose chemotherapy with stem cell transplant: review and consensus statement. Support Care Cancer. 2005;13:112–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, X., Huang, J., Cao, R. et al. Palonosetron for prevention of acute and delayed nausea and vomiting in non-small-cell lung carcinoma patients. Med Oncol 28, 1425–1429 (2011). https://doi.org/10.1007/s12032-010-9608-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-010-9608-y