Abstract
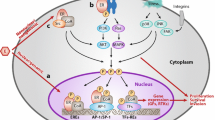
Endocrine therapy (ET) with aromatase inhibitors (AIs) has become the standard of care for postmenopausal women with hormone-receptor–positive (HR+) advanced breast cancer (ABC); however, progression following initial treatment remains a major clinical challenge given the large patient population, many of whom develop progressive disease. There is an unmet need for treatment strategies that can overcome endocrine resistance. Growth factor-mediated signaling pathways, such as the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, contribute to estrogen-independent growth that may lead to endocrine resistance. Preclinical studies have demonstrated that the use of mTOR inhibitors, such as everolimus and temsirolimus, is a promising strategy to potentially enhance endocrine sensitivity in ABC. This review will focus on the current ET options for women with HR+ ABC who have progressed on prior AI therapy, the role of mTOR-mediated signaling in breast cancer, and the clinical evidence supporting the use of mTOR inhibitors.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9 Suppl 1:S6–S17.
Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–803.
• Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–52. The 1st international Consensus Conference for Advanced Breast Cancer (ABC 1) took place in November 2011 in Lisbon, Portugal. This manuscript summarizes the consensus treatment guidelines developed by this international expert panel specifically for the management of ABC.
• National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version I. 2012. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 8 June 2012. The latest version of the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Breast Cancer recommends considering the addition of everolimus to exemestane in women who fulfill the eligibility criteria of the Phase 3 BOLERO-2 study.
Hurvitz SA, Pietras RJ. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113:2385–97.
Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600.
Johnston S, Kilburn LS, Ellis P, et al. Fulvestrant alone or with concomitant anastrozole vs exemestane following progression on non-steroidal aromatase inhibitor: first results of the SoFEA trial (CRUKE/03/021 & CRUK/09/007) (ISRCTN44195747) [Abstract 2LBA]. Eur J Cancer. 2012;48:2s.
Johnston SR. BOLERO-2 - will this change practice in advanced breast cancer? Breast Cancer Res. 2012;14:311.
• Villarreal-Garza C, Cortes J, Andre F, Verma S. mTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directions. Ann Oncol. 2012; [Epub ahead of print]. Recent review evaluating the use of mTOR inhibitors in the treatment of HR + BC.
Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47.
Margariti N, Fox SB, Bottini A, Generali D. “Overcoming breast cancer drug resistance with mTOR inhibitors”. Could it be a myth or a real possibility in the short-term future? Breast Cancer Res Treat. 2011;128:599–606.
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30.
Wu G, Xing M, Mambo E, et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005;7:R609–16.
Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5.
Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9.
Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–10.
Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13.
de Graffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10:8059–67.
Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28.
Beeram M, Tan QT, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–8.
Wyeth Pharmaceuticals Inc, a subsidiary of Pfizer, Inc, Philadelphia, PA. Torisel (temsirolimus) prescribing information. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022088s014lbl.pdf. Accessed 28 June 2012.
Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–22.
Baselga J, Roche H, Fumoleau P. Treatment of postmenopausal women with locally advanced or metastatic breast cancer with letrozole alone or in combination with temsirolimus: a randomized, 3-arm, phase 2 study [abstract 1068]. Breast Cancer Res Treat. 2005;94:S62.
Chow LWC, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer [abstract 6091]. Breast Cancer Res Treat. 2006;100:S286.
Novartis Pharmaceuticals Corporation, East Hanover, NJ. Afinitor (everolimus) prescribing information. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022334s016lbl.pdf. Accessed 8 August 2012.
Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91.
Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–41.
Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7.
• Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–24. The phase 2 TAMRAD study demonstrated that addition of everolimus to ET improves clinical benefit compared with ET alone in PMW with HR + , HER2 − , AI-resistant metastatic BC.
•• Hortobagyi GN, Piccart M, Rugo H, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO-2 phase III trial [abstract S3-7]. Cancer Res. 2011;71:105s–6s. Interim PFS analysis from the BOLERO-2 study demonstrated a robust and consistent benefit of everolimus plus exemestane in HR + ABC patients.
Bhattacharyya GS, Biswas J, Singh JK, et al. Reversal of tamoxifen resistance (hormone resistance) by addition of sirolimus (mTOR inhibitor) in metastatic breast cancer [abstract 16LBA]. Presented at the European Multidisciplinary Cancer Congress. Stockholm, Sweden; 2011.
Rugo HS, Keck S. Reversing hormone resistance: have we found the golden key? J Clin Oncol. 2012;30:2707–9.
•• Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. BOLERO-2 was the first phase 3 study to demonstrate that addition of an mTOR inhibitor, specifically everolimus, to an AI improves clinical benefit compared with AI therapy alone in PMW with HR + , HER2 − , AI-resistant ABC.
Piccart M, Noguchi S, Pritchard KI, et al. Everolimus for postmenopausal women with advanced breast cancer: Updated results of the BOLERO-2 phase III trial [abstract 559]. Presented at the 48th Annual Meeting of the American Society of Clinical Oncology. Chicago, IL; 2012.
• Gnant M, Baselga J, Rugo HS, et al. Effects of everolimus (EVE) on disease progression in bone and bone markers (BM) in patients (pts) with bone metastases (mets) [abstract 512]. Presented at the 48th Annual Meeting of the American Society of Clinical Oncology. Chicago, IL; 2012. Exploratory analyses from the BOLERO-2 study demonstrated that in patients with bone metastases, addition of everolimus decreased markers of bone turnover compared with exemestane alone.
Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81.
Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65.
• Porta C, Osanto S, Ravaud A, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47:1287–98. Recommendations from an expert panel for AE management during everolimus treatment for patients with RCC; these recommendations are also applicable to the BC setting.
Afinitor (everolimus) US Prescribing Information July 20. 2012. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022334s016lbl.pdf. Accessed 23 July 2012.
Sonis S, Treister N, Chawla S, et al. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210–5.
de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:998–1003.
Stokman MA, Spijkervet FK, Boezen HM, et al. Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: results of meta-analyses. J Dent Res. 2006;85:690–700.
Potting CM, Uitterhoeve R, Op Reimer WS, Van Achterberg T. The effectiveness of commonly used mouthwashes for the prevention of chemotherapy-induced oral mucositis: a systematic review. Eur J Cancer Care (Engl). 2006;15:431–9.
Harris DJ, Eilers J, Harriman A, et al. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs. 2008;12:141–52.
Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006;42:1875–80.
Cho D, Signoretti S, Regan M, et al. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–63s.
White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10.
Buckner JC, Forouzesh B, Erlichman C, et al. Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs. 2010;28:334–42.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clin Cancer Res. 2003;9:5078–84.
Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27.
Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402.
Andre F, Campone M, O’Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5.
Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–55.
• Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–32. This pooled analysis of 2 phase 1/2 studies suggests that everolimus in combination with trastuzumab provides clinical benefit in patients with trastuzumab-resistant, HER2 + metastatic BC.
Hurvitz SA, Andre F, Burris HA, et al. BOLERO-1: A randomized, phase III, double-blind, placebo-controlled multicenter trial of everolimus in combination with trastuzumab and paclitaxel as first-line therapy in women with HER2-positive (HER2+), locally advanced or metastatic breast cancer (BC) [abstract TPS648]. J Clin Oncol. 2012;30:43s.
Daily everolimus in combination with trastuzumab and vinorelbine in HER2/Neu positive women with locally advanced or metastatic breast cancer (BOLERO-3). Available at http://clinicaltrials.gov/ct2/show/NCT01007942. Accessed 1 May 2012.
Novartis Pharma GmbH, Nuremberg, Germany. Afinitor (everolimus) EPAR - product information. 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf. Accessed 8 August 2012.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. I thank Payal N. Gandhi, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
Disclosure
M. Gnant: consultancy fees (Novartis, Merrion), speaker’s fees (Roche, Amgen, Novartis, Astra Zeneca, GlaxoSmithKline), and unrestricted grant/research funding (Roche, Sanofi–Aventis, GlaxoSmithKline, Novartis, AstraZeneca).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gnant, M. The Role of Mammalian Target of Rapamycin (mTOR) Inhibition in the Treatment of Advanced Breast Cancer. Curr Oncol Rep 15, 14–23 (2013). https://doi.org/10.1007/s11912-012-0277-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-012-0277-1