Summary
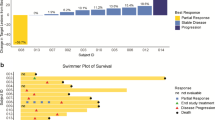
Introduction Drugs inhibiting the mammalian target of rapamycin (mTOR) are approved in the treatment of renal cell carcinoma (RCC), but resistance inevitably emerges. Proposed escape pathways include increased phosphorylation of Akt, which can be down regulated by histone deacetylase (HDAC) inhibitors. We hypothesized that co-treatment with the mTOR inhibitor ridaforolimus and the HDAC inhibitor vorinostat may abrogate resistance in RCC. Methods This phase 1 study evaluated the co-administration of ridaforolimus and vorinostat in patients with advanced solid tumors. The primary objective was to determine the maximum tolerated dose (MTD) in RCC patients. Although all solid tumors were allowed, prior cytotoxic chemotherapy was limited to 1 regimen. Using a modified 3 + 3 dose escalation design, various dose combinations were tested concurrently in separate cohorts. Efficacy was a secondary endpoint. Results Fifteen patients were treated at one of three dose levels, thirteen with RCC (10 clear cell, 3 papillary). Dosing was limited by thrombocytopenia. The MTD was determined to be ridaforolimus 20 mg daily days 1–5 with vorinostat 100 mg BID days 1–3 weekly, however late onset thrombocytopenia led to a lower recommended phase II dose: ridaforolimus 20 mg daily days 1–5 with vorinostat 100 mg daily days 1–3 weekly. Two patients, both with papillary RCC, maintained disease control for 54 and 80 weeks, respectively. Conclusions The combination of ridaforolimus and vorinostat was tolerable at the recommended phase II dose. Two patients with papillary RCC experienced prolonged disease stabilization, thus further study of combined HDAC and mTOR inhibition in this population is warranted.





Similar content being viewed by others
References
Chan S (2004) Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 91(8):1420–1424
Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441(7092):424–430
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103(2):253–262
Robb VA et al (2007) Activation of the mTOR signaling pathway in renal clear cell carcinoma. J Urol 177(1):346–352
Mills GB et al (2001) The role of genetic abnormalities of PTEN and the phosphatidylinositol 3-kinase pathway in breast and ovarian tumorigenesis, prognosis, and therapy. Semin Oncol 28(5 Suppl 16):125–141
Barbet NC et al (1996) TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell 7(1):25–42
Hay N, Sonenberg N (2004) Upstream and downstream of mTOR. Genes Dev 18(16):1926–1945
Pantuck AJ et al (2003) Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res 9(13):4641–4652
Thomas GV et al (2006) Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med 12(1):122–127
Hudes G et al (2007) Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356(22):2271–2281
Motzer RJ et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372(9637):449–456
Treins C et al (2002) Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277(31):27975–27981
Raval RR et al (2005) Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol 25(13):5675–5686
Clifford SC et al (1998) Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: Evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosom Cancer 22(3):200–209
Hudes GR (2009) Targeting mTOR in renal cell carcinoma. Cancer 115(S10):2313–2320
O’Reilly KE et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66(3):1500–1508
Verheul HM et al (2008) Combination strategy targeting the hypoxia inducible factor-1 alpha with mammalian target of rapamycin and histone deacetylase inhibitors. Clin Cancer Res 14(11):3589–3597
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5(9):769–784
Kelly WK et al (2005) Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23(17):3923–3931
Matsuoka H et al (2007) Mechanisms of HDAC inhibitor-induced thrombocytopenia. Eur J Pharmacol 571(2):88–96
Mita MM et al (2008) Phase I trial of the novel mammalian target of rapamycin inhibitor deforolimus (AP23573; MK-8669) administered intravenously daily for 5 days every 2 weeks to patients with advanced malignancies. J Clin Oncol 26(3):361–367
Gupta M et al (2009) Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood 114(14):2926–2935
Morgan S, Cranmer L Effect of the combination of mTOR inhibitor ridaforolimus and HDAC inhibitor vorinostat on in vitro synergism in synovial sarcoma, osteosarcoma, and a range of other tumor subtypes. In Journal Of Clinical Oncology. 2011. Amer Soc Clinical Oncology 2318 mill road, STE 800, Alexandria, VA 22314 USA.
Wedel S et al (2011) Inhibitory effects of the HDAC inhibitor valproic acid on prostate cancer growth are enhanced by simultaneous application of the mTOR inhibitor RAD001. Life Sci 88(9):418–424
Huang X et al (2007) A parallel phase I/II clinical trial design for combination therapies. Biometrics 63(2):429–436
Confidential Investigators Brochure: Vorinostat, I. Merck and Co., Editor
Dutcher JP et al (2009) Effect of temsirolimus versus interferon-alpha on outcome of patients with advanced renal cell carcinoma of different tumor histologies. Med Oncol 26(2):202–209
Chaux A et al (2012) Immunoexpression status and prognostic value of mammalian target of rapamycin and hypoxia-induced pathway members in papillary cell renal cell carcinomas. Hum Pathol 43(12):2129–2137
Acknowledgments
The authors would like to thank the patients who enrolled in this trial, as well as their families
Funding Sources
This work was supported in part by Merck Inc.
Financial Disclosures
ERP has served as a consultant for Merck Inc.
Precis for use in the Table of Contents
HDAC inhibition may abrogate Akt mediated resistance to mTOR inhibition. When combined in this phase I trial, the mTOR inhibitor ridaforolimus and the HDAC inhibitor vorinostat were safe in combination and preliminary efficacy results suggest proof of concept.
Author Contributions
Conception and Design: ERP
Acquisition of data: YNW, LM, AJO, CSD, SKR, CHT, ERP.
Analysis and interpretation of data: MZ, KD, and ERP.
Writing, review and/or revision of the manuscript: All authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration ID: NCT01169532
Neither the submitted manuscript nor any similar manuscript, in whole or in part, other than an abstract, is under consideration, in press, published, or reported elsewhere.
Rights and permissions
About this article
Cite this article
Zibelman, M., Wong, YN., Devarajan, K. et al. Phase I study of the mTOR inhibitor ridaforolimus and the HDAC inhibitor vorinostat in advanced renal cell carcinoma and other solid tumors. Invest New Drugs 33, 1040–1047 (2015). https://doi.org/10.1007/s10637-015-0261-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0261-3