Abstract
Background
This study investigated the effects of the receptor activator for nuclear factor κB ligand (RANKL) inhibitor, osteoprotegerin (OPG), on tumor-induced allodynia, osteolysis, and bone histology in the mammary tumor (MRMT-1) rat model for bone cancer pain.
Methods
Rats (n = 8/group) were inoculated with MRMT-1 or culture medium in the proximal right tibia, injected with OPG or vehicle subcutaneously 2–3 times weekly, evaluated for mechanical allodynia with von Frey paw stimulation, and euthanized on Day 20 for necropsy. Three groups were evaluated starting on Day 5 and received the following interventions beginning on Day 1: tumor and OPG, tumor and vehicle, or culture medium and vehicle. Three additional groups received the same interventions but were evaluated starting on Day 3. A seventh group started OPG on Day 8 after tumor inoculation.
Results
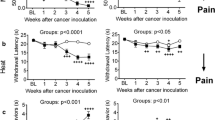
Starting OPG on Day 1 reduced allodynia significantly compared with vehicle injections; pain relief was observed within 5–6 days after tumor inoculation and lasted throughout follow-up. Starting OPG on Day 8 did not reverse allodynia significantly compared with the tumor control group. Regardless of treatment start time, OPG treatment reduced osteoclast number and tartrate-resistant acid phosphatase levels, increased bone mineral density, preserved normal bone volume and integrity on micro-computed tomography, reduced relative tumor volume in the bone, and reduced staining for glial fibrillary acidic protein in the spinal cord.
Conclusions
RANKL inhibition with OPG reduced bone resorption and bone pain in rats with malignant bone disease; further study is warranted to determine if RANKL inhibition has similar benefits in humans.
Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BMD:
-
Bone mineral density
- DXA:
-
Dual X-ray absorptiometry
- GFAP:
-
Glial fibrillary acidic protein
- MicroCT:
-
Micro-computed tomography
- MRMT-1:
-
Rat mammary tumor cell line
- OPG:
-
Osteoprotegerin
- RANK:
-
Receptor activator for nuclear factor κB
- RANKL:
-
Receptor activator for nuclear factor κB ligand
- sTRAP5b:
-
Tartrate-resistant acid phosphatase form 5b
References
Rubens RD (1998) Bone metastases – the clinical problem. Eur J Cancer 34(2):210–213
Mercadante S (1997) Malignant bone pain: pathophysiology and treatment. Pain 69(1–2):1–18
Schwei MJ, Honore P, Rogers SD et al (1999) Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 19(24):10886–10897
Honore P, Rogers SD, Schwei MJ et al (2000) Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98(3):585–598
Cain DM, Wacnik PW, Turner M et al (2001) Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci 21(23):9367–9376
Medhurst SJ, Walker K, Bowes M et al (2002) A rat model of bone cancer pain. Pain 96(1–2):129–140
Clohisy DR, Mantyh PW (2004) Bone cancer pain and the role of RANKL/OPG. J Musculoskelet Neuronal Interact 4(3):293–300
Blair JM, Zhou H, Seibel MJ et al (2006) Mechanisms of disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nat Clin Pract Oncol 3(1):41–49
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Yasuda H, Shima N, Nakagawa N et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 95(7):3597–3602
Burgess TL, Qian Y, Kaufman S et al (1999) The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol 145(3):527–538
Hsu H, Lacey DL, Dunstan CR et al (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA 96(7):3540–3545
Kong YY, Yoshida H, Sarosi I et al (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph node organogenesis. Nature 397:315–323
Lacey DL, Tan HL, Lu J et al (2000) Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 157(2):435–448
Simonet WS, Lacey DL, Dunstan CR et al (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89(2):309–319
Udagawa N, Takahashi N, Yasuda H et al (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Urch C (2004) The pathophysiology of cancer-induced bone pain: current understanding. Palliat Med 18(4):267–274
Lipton A, Ali SM, Leitzel K et al (2002) Serum osteoprotegerin levels in healthy controls and cancer patients. Clin Cancer Res 8(7):2306–2310
Terpos E, Szydlo R, Apperley JF et al (2003) Soluble receptor activator of nuclear factor kappaB ligand–osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102(3):1064–1069
Grimaud E, Soubigou L, Couillaud S et al (2003) Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am J Pathol 163(5):2021–2031
Capparelli C, Morony S, Warmington K et al (2003) Sustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats. J Bone Miner Res 18:852–858
Clohisy DR, Ramnaraine ML, Scully S et al (2000) Osteoprotegerin inhibits tumor-induced osteoclastogenesis and bone tumor growth in osteopetrotic mice. J Orthop Res 18(6):967–976
Morony S, Capparelli C, Sarosi I et al (2001) Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61(11):4432–4436
Honore P, Luger NM, Sabino MA et al (2000) Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 6(5):521–528
Luger NM, Honore P, Sabino MA et al (2001) Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res 61(10):4038–4047
Chaplan SR, Bach FW, Pogrel JW et al (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Olson TH, Riedl MS, Vulchanova L et al (1998) An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport 9(6):1109–1113
Li Y, Kucuk O, Hussain M et al (2006) Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res 66(9):4816–4825
Body JJ, Facon T, Coleman RE et al (2006) A study of the biologic RANKL inhibitor, denosumab (AMG 162), in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 12(4):1221–1228
Li EC, Davis LE (2003) Zoledronic acid: a new parenteral bisphosphonate. Clin Ther 25(11):2669–2708
Walker K, Medhurst SJ, Kidd BL et al (2002) Disease modifying and anti-nociceptive effects of the bisphosphonate, zoledronic acid in a model of bone cancer pain. Pain 100(3):219–229
Bonabello A, Galmozzi MR, Canaparo R et al (2003) Long-term analgesic effect of clodronate in rodents. Bone 33(4):567–574
Sevcik MA, Luger NM, Mach DB et al (2004) Bone cancer pain: the effects of the bisphosphonate alendronate on pain, skeletal remodeling, tumor growth and tumor necrosis. Pain 111(1–2):169–180
Goicoechea C, Porras E, Alfaro MJ et al (1999) Alendronate induces antinociception in mice, not related with its effects in bone. Jpn J Pharmacol 79(4):433–437
Acknowledgments
This study was supported by a grant from Amgen, Inc. At the time of the study, William C. Dougall was an employee of Amgen, Inc., which is researching and developing an anti-RANKL monoclonal antibody for clinical use, and Martine Roudier and Steve Bain were employees of SkeleTech, Inc., which received a grant from Amgen, Inc. to perform the study. Chung Liu, Hellen Zheng, and Eric Bell provided technical assistance. Jonathan N. Latham and Christine Gatchalian provided editorial assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roudier, M.P., Bain, S.D. & Dougall, W.C. Effects of the RANKL inhibitor, osteoprotegerin, on the pain and histopathology of bone cancer in rats. Clin Exp Metastasis 23, 167–175 (2006). https://doi.org/10.1007/s10585-006-9026-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-006-9026-x