Abstract
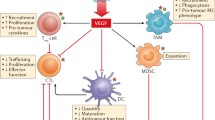
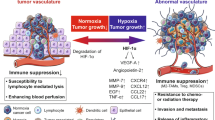
The immune system regulates angiogenesis in cancer with both pro- and antiangiogenic activities. The induction of angiogenesis is mediated by tumor-associated macrophages and myeloid-derived suppressor cells (MDSC) which produce proinflammatory cytokines, endothelial growth factors (VEGF, bFGF…), and protease (MMP9) implicated in neoangiogenesis. Some cytokines (IL-6, IL-17…) activated Stat3 which also led to the production of VEGF and bFGF. In contrast, other cytokines (IFN, IL-12, IL-21, and IL-27) display an antiangiogenic activity. Recently, it has been shown that some antiangiogenic molecules alleviates immunosuppression associated with cancer by decreasing immunosuppressive cells (MDSC, regulatory T cells), immunosuppressive cytokines (IL-10, TGFβ), and inhibitory molecules on T cells (PD-1). Some of these broad effects may result from the ability of some antiangiogenic molecules, especially cytokines to inhibit the Stat3 transcription factor. The association often observed between angiogenesis and immunosuppression may be related to hypoxia which induces both neoangiogenesis via activation of HIF-1 and VEGF and favors the intratumor recruitment and differentiation of regulatory T cells and MDSC. Preliminary studies suggest that modulation of immune markers (intratumoral MDSC and IL-8, peripheral regulatory T cells…) may predict clinical response to antiangiogenic therapy. In preclinical models, a synergy has been observed between antiangiogenic molecules and immunotherapy which may be explained by an improvement of immune status in tumor-bearing mice after antiangiogenic therapy. In preclinical models, antiangiogenic molecules promoted intratumor trafficking of effector cells, enhance endogenous anti-tumor response, and synergyzed with immunotherapy protocols to cure established murine tumors. All these results warrant the development of clinical trials combining antiangiogenic drugs and immunotherapy.

Similar content being viewed by others
References
Oudard, S., George, D., Medioni, J., & Motzer, R. (2007). Treatment options in renal cell carcinoma: Past, present and future. Annals of Oncology, 18(Suppl 10), x25–x31.
Rini, B. I., Campbell, S. C., & Escudier, B. (2009). Renal cell carcinoma. Lancet, 373, 1119–1132.
Oudard, S., Medioni, J., Aylllon, J., Barrascourt, E., Elaidi, R. T., Balcaceres, J., et al. (2009). Everolimus (RAD001): An mTOR inhibitor for the treatment of metastatic renal cell carcinoma. Expert Review of Anticancer Therapy, 9, 705–717.
Motzer, R. J., Hutson, T. E., Tomczak, P., Michaelson, M. D., Bukowski, R. M., Rixe, O., et al. (2007). Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. The New England Journal of Medicine, 356, 115–124.
Barrascout, E., Medioni, J., Scotte, F., Ayllon, J., Mejean, A., Cuenod, C. A., et al. (2010). Angiogenesis inhibition: Review of the activity of sorafenib, sunitinib and bevacizumab. Bulletin du Cancer, 97, 29–43.
Escudier, B., Bellmunt, J., Negrier, S., Bajetta, E., Melichar, B., Bracarda, S., et al. (2010). Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): Final analysis of overall survival. Journal of Clinical Oncology, 28, 2144–2150.
Rini, B. I., Halabi, S., Rosenberg, J. E., Stadler, W. M., Vaena, D. A., Archer, L., et al. (2010). Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. Journal of Clinical Oncology, 28, 2137–2143.
Motzer, R. J., Escudier, B., Oudard, S., Hutson, T. E., Porta, C., Bracarda, S., et al. (2008). Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet, 372, 449–456.
Van Meter, M. E., & Kim, E. S. (2010). Bevacizumab: Current updates in treatment. Current Opinion in Oncology, 22, 586–591.
Paez-Ribes, M., Allen, E., Hudock, J., Takeda, T., Okuyama, H., Vinals, F., et al. (2009). Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell, 15, 220–231.
Ebos, J. M., Lee, C. R., Cruz-Munoz, W., Bjarnason, G. A., Christensen, J. G., & Kerbel, R. S. (2009). Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell, 15, 232–239.
Rini, B. I., & Flaherty, K. (2008). Clinical effect and future considerations for molecularly-targeted therapy in renal cell carcinoma. Urologic Oncology, 26, 543–549.
Albini, A., Tosetti, F., Benelli, R., & Noonan, D. M. (2005). Tumor inflammatory angiogenesis and its chemoprevention. Cancer Research, 65, 10637–10641.
de Visser, K. E., Eichten, A., & Coussens, L. M. (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews. Cancer, 6, 24–37.
Murdoch, C., Muthana, M., Coffelt, S. B., & Lewis, C. E. (2008). The role of myeloid cells in the promotion of tumour angiogenesis. Nature Reviews. Cancer, 8, 618–631.
Sunderkotter, C., Steinbrink, K., Goebeler, M., Bhardwaj, R., & Sorg, C. (1994). Macrophages and angiogenesis. Journal of Leukocyte Biology, 55, 410–422.
Kimura, Y. N., Watari, K., Fotovati, A., Hosoi, F., Yasumoto, K., Izumi, H., et al. (2007). Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Science, 98, 2009–2018.
Lewis, C. E., Leek, R., Harris, A., & McGee, J. O. (1995). Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. Journal of Leukocyte Biology, 57, 747–751.
Mantovani, A., Schioppa, T., Porta, C., Allavena, P., & Sica, A. (2006). Role of tumor-associated macrophages in tumor progression and invasion. Cancer and Metastasis Reviews, 25, 315–322.
Grunewald, M., Avraham, I., Dor, Y., Bachar-Lustig, E., Itin, A., Jung, S., et al. (2006). VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell, 124, 175–189.
Du, R., Lu, K. V., Petritsch, C., Liu, P., Ganss, R., Passegue, E., et al. (2008). HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell, 13, 206–220.
Giraudo, E., Inoue, M., & Hanahan, D. (2004). An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. The Journal of Clinical Investigation, 114, 623–633.
Neufeld, G., & Kessler, O. (2006). Pro-angiogenic cytokines and their role in tumor angiogenesis. Cancer and Metastasis Reviews, 25, 373–385.
Kim, Y. M., Lee, Y. M., Kim, H. S., Kim, J. D., Choi, Y., Kim, K. W., et al. (2002). TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. The Journal of Biological Chemistry, 277, 6799–6805.
Silva-Santos, B. (2010). Promoting angiogenesis within the tumor microenvironment: The secret life of murine lymphoid IL-17-producing gammadelta T cells. European Journal of Immunology, 40, 1873–1876.
Numasaki, M., Fukushi, J., Ono, M., Narula, S. K., Zavodny, P. J., Kudo, T., et al. (2003). Interleukin-17 promotes angiogenesis and tumor growth. Blood, 101, 2620–2627.
Zou, W., & Restifo, N. P. (2010). T(H)17 cells in tumour immunity and immunotherapy. Nature Reviews. Immunology, 10, 248–256.
Numasaki, M., Watanabe, M., Suzuki, T., Takahashi, H., Nakamura, A., McAllister, F., et al. (2005). IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. Journal of Immunology, 175, 6177–6189.
Ciree, A., Michel, L., Camilleri-Broet, S., Jean Louis, F., Oster, M., Flageul, B., et al. (2004). Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome). International Journal of Cancer, 112, 113–120.
Pickens, S. R., Volin, M. V., Mandelin, A. M., 2nd, Kolls, J. K., Pope, R. M., & Shahrara, S. (2010). IL-17 contributes to angiogenesis in rheumatoid arthritis. Journal of Immunology, 184, 3233–3241.
Kuniyasu, H., Ohmori, H., Sasaki, T., Sasahira, T., Yoshida, K., Kitadai, Y., et al. (2003). Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clinical Cancer Research, 9, 4802–4810.
Badoual, C., Bouchaud, G., Agueznay Nel, H., Mortier, E., Hans, S., Gey, A., et al. (2008). The soluble alpha chain of interleukin-15 receptor: A proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Research, 68, 3907–3914.
Yu, H., Kortylewski, M., & Pardoll, D. (2007). Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nature Reviews. Immunology, 7, 41–51.
Badoual, C., Sandoval, F., Pere, H., Hans, S., Gey, A., Merillon, N., et al. (2010). Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head & Neck, 32, 946–958.
Voest, E. E., Kenyon, B. M., O’Reilly, M. S., Truitt, G., D’Amato, R. J., & Folkman, J. (1995). Inhibition of angiogenesis in vivo by interleukin 12. Journal of the National Cancer Institute, 87, 581–586.
Sgadari, C., Angiolillo, A. L., & Tosato, G. (1996). Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood, 87, 3877–3882.
Haicheur, N., Escudier, B., Dorval, T., Negrier, S., De Mulder, P. H., Dupuy, J. M., et al. (2000). Cytokines and soluble cytokine receptor induction after IL-12 administration in cancer patients. Clinical and Experimental Immunology, 119, 28–37.
Dias, S., Boyd, R., & Balkwill, F. (1998). IL-12 regulates VEGF and MMPs in a murine breast cancer model. International Journal of Cancer, 78, 361–365.
Dinney, C. P., Bielenberg, D. R., Perrotte, P., Reich, R., Eve, B. Y., Bucana, C. D., et al. (1998). Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Research, 58, 808–814.
Mitola, S., Strasly, M., Prato, M., Ghia, P., & Bussolino, F. (2003). IL-12 regulates an endothelial cell-lymphocyte network: Effect on metalloproteinase-9 production. Journal of Immunology, 171, 3725–3733.
Shimizu, M., Shimamura, M., Owaki, T., Asakawa, M., Fujita, K., Kudo, M., et al. (2006). Antiangiogenic and antitumor activities of IL-27. Journal of Immunology, 176, 7317–7324.
Castermans, K., Tabruyn, S. P., Zeng, R., van Beijnum, J. R., Eppolito, C., Leonard, W. J., et al. (2008). Angiostatic activity of the antitumor cytokine interleukin-21. Blood, 112, 4940–4947.
Tartour, E., Mathiot, C., & Fridman, W. H. (1992). Current status of interleukin-2 therapy in cancer. Biomedicine & Pharmacotherapy, 46, 473–484.
Ellis, L. M., & Hicklin, D. J. (2008). VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nature Reviews. Cancer, 8, 579–591.
Belkaid, Y., & Oldenhove, G. (2008). Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity, 29, 362–371.
Curiel, T. J. (2007). Tregs and rethinking cancer immunotherapy. The Journal of Clinical Investigation, 117, 1167–1174.
Oyama, T., Ran, S., Ishida, T., Nadaf, S., Kerr, L., Carbone, D. P., et al. (1998). Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. Journal of Immunology, 160, 1224–1232.
Dikov, M. M., Ohm, J. E., Ray, N., Tchekneva, E. E., Burlison, J., Moghanaki, D., et al. (2005). Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. Journal of Immunology, 174, 215–222.
Gabrilovich, D. I., Ishida, T., Nadaf, S., Ohm, J. E., & Carbone, D. P. (1999). Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clinical Cancer Research, 5, 2963–2970.
Batchelor, T. T., Sorensen, A. G., di Tomaso, E., Zhang, W. T., Duda, D. G., Cohen, K. S., et al. (2007). AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell, 11, 83–95.
Laxmanan, S., Robertson, S. W., Wang, E., Lau, J. S., Briscoe, D. M., & Mukhopadhyay, D. (2005). Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochemical and Biophysical Research Communications, 334, 193–198.
Ohm, J. E., Shurin, M. R., Esche, C., Lotze, M. T., Carbone, D. P., & Gabrilovich, D. I. (1999). Effect of vascular endothelial growth factor and FLT3 ligand on dendritic cell generation in vivo. Journal of Immunology, 163, 3260–3268.
Gabrilovich, D. I., Chen, H. L., Girgis, K. R., Cunningham, H. T., Meny, G. M., Nadaf, S., et al. (1996). Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Natural Medicines, 2, 1096–1103.
Ishida, T., Oyama, T., Carbone, D. P., & Gabrilovich, D. I. (1998). Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. Journal of Immunology, 161, 4842–4851.
Osada, T., Chong, G., Tansik, R., Hong, T., Spector, N., Kumar, R., et al. (2008). The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunology, Immunotherapy, 57, 1115–1124.
Fricke, I., Mirza, N., Dupont, J., Lockhart, C., Jackson, A., Lee, J. H., et al. (2007). Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clinical Cancer Research, 13, 4840–4848.
Strauss, L., Volland, D., Kunkel, M., & Reichert, T. E. (2005). Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): Possible link between angiogenesis and immune tolerance. Medical Science Monitor, 11, BR280–292.
Almand, B., Resser, J. R., Lindman, B., Nadaf, S., Clark, J. I., Kwon, E. D., et al. (2000). Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research, 6, 1755–1766.
Boissel, N., Rousselot, P., Raffoux, E., Cayuela, J. M., Maarek, O., Charron, D., et al. (2004). Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia, 18, 1656–1661.
Finke, J. H., Rini, B., Ireland, J., Rayman, P., Richmond, A., Golshayan, A., et al. (2008). Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clinical Cancer Research, 14, 6674–6682.
Hipp, M. M., Hilf, N., Walter, S., Werth, D., Brauer, K. M., Radsak, M. P., et al. (2008). Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood, 111, 5610–5620.
Adotevi, O., Pere, H., Ravel, P., Haicheur, N., Badoual, C., Merillon, N., et al. (2010). A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J Immunother, 33, 991–998.
Abe, F., Younos, I., Westphal, S., Samson, H., Scholar, E., Dafferner, A., et al. (2010). Therapeutic activity of sunitinib for Her2/neu induced mammary cancer in FVB mice. International Immunopharmacology, 10, 140–145.
Badoual, C., Hans, S., Rodriguez, J., Peyrard, S., Klein, C., Agueznay Nel, H., et al. (2006). Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clinical Cancer Research, 12, 465–472.
Badoual, C., Hans, S., Fridman, W. H., Brasnu, D., Erdman, S., & Tartour, E. (2009). Revisiting the prognostic value of regulatory T cells in patients with cancer. Journal of Clinical Oncology, 27, e5–6. author reply e7.
Suzuki, H., Onishi, H., Wada, J., Yamasaki, A., Tanaka, H., Nakano, K., et al. (2010). VEGFR2 is selectively expressed by FOXP3high CD4+ Treg. European Journal of Immunology, 40, 197–203.
Ko, J. S., Zea, A. H., Rini, B. I., Ireland, J. L., Elson, P., Cohen, P., et al. (2009). Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clinical Cancer Research, 15, 2148–2157.
Huang, B., Pan, P. Y., Li, Q., Sato, A. I., Levy, D. E., Bromberg, J., et al. (2006). Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Research, 66, 1123–1131.
Gabrilovich, D., Ishida, T., Oyama, T., Ran, S., Kravtsov, V., Nadaf, S., et al. (1998). Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood, 92, 4150–4166.
Ohm, J. E., & Carbone, D. P. (2001). VEGF as a mediator of tumor-associated immunodeficiency. Immunologic Research, 23, 263–272.
Shojaei, F., Wu, X., Zhong, C., Yu, L., Liang, X. H., Yao, J., et al. (2007). Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature, 450, 825–831.
Pan, P. Y., Wang, G. X., Yin, B., Ozao, J., Ku, T., Divino, C. M., et al. (2008). Reversion of immune tolerance in advanced malignancy: Modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood, 111, 219–228.
Curti, A., Fogli, M., Ratta, M., Tura, S., & Lemoli, R. M. (2001). Stem cell factor and FLT3-ligand are strictly required to sustain the long-term expansion of primitive CD34+DR- dendritic cell precursors. Journal of Immunology, 166, 848–854.
Filipazzi, P., Valenti, R., Huber, V., Pilla, L., Canese, P., Iero, M., et al. (2007). Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. Journal of Clinical Oncology, 25, 2546–2553.
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology, 9, 162–174.
Serafini, P., Carbley, R., Noonan, K. A., Tan, G., Bronte, V., & Borrello, I. (2004). High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Research, 64, 6337–6343.
Marigo, I., Dolcetti, L., Serafini, P., Zanovello, P., & Bronte, V. (2008). Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological Reviews, 222, 162–179.
Costantino, L., & Barlocco, D. (2008). STAT 3 as a target for cancer drug discovery. Current Medicinal Chemistry, 15, 834–843.
Kortylewski, M., Kujawski, M., Wang, T., Wei, S., Zhang, S., Pilon-Thomas, S., et al. (2005). Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Natural Medicines, 11, 1314–1321.
Kortylewski, M., & Yu, H. (2008). Role of Stat3 in suppressing anti-tumor immunity. Current Opinion in Immunology, 20, 228–233.
Yu, H., & Jove, R. (2004). The STATs of cancer—New molecular targets come of age. Nature Reviews. Cancer, 4, 97–105.
Xie, T. X., Wei, D., Liu, M., Gao, A. C., Ali-Osman, F., Sawaya, R., et al. (2004). Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene, 23, 3550–3560.
Noman, M. Z., Buart, S., Van Pelt, J., Richon, C., Hasmim, M., Leleu, N., et al. (2009). The cooperative induction of hypoxia-inducible factor-1alpha and STAT3 during hypoxia induced an impairment of tumor susceptibility to CTL-mediated cell lysis. Journal of Immunology, 182, 3510–3521.
Kujawski, M., Kortylewski, M., Lee, H., Herrmann, A., Kay, H., & Yu, H. (2008). Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. The Journal of Clinical Investigation, 118, 3367–3377.
Xin, H., Zhang, C., Herrmann, A., Du, Y., Figlin, R., & Yu, H. (2009). Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Research, 69, 2506–2513.
Kim, D. W., Jo, Y. S., Jung, H. S., Chung, H. K., Song, J. H., Park, K. C., et al. (2006). An orally administered multitarget tyrosine kinase inhibitor, SU11248, is a novel potent inhibitor of thyroid oncogenic RET/papillary thyroid cancer kinases. The Journal of Clinical Endocrinology and Metabolism, 91, 4070–4076.
Ozao-Choy, J., Ma, G., Kao, J., Wang, G. X., Meseck, M., Sung, M., et al. (2009). The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Research, 69, 2514–2522.
Inman, B. A., Frigola, X., Dong, H., & Kwon, E. D. (2007). Costimulation, coinhibition and cancer. Current Cancer Drug Targets, 7, 15–30.
Fox, S. B., Launchbury, R., Bates, G. J., Han, C., Shaida, N., Malone, P. R., et al. (2007). The number of regulatory T cells in prostate cancer is associated with the androgen receptor and hypoxia-inducible factor (HIF)-2alpha but not HIF-1alpha. The Prostate, 67, 623–629.
Aghi, M., Cohen, K. S., Klein, R. J., Scadden, D. T., & Chiocca, E. A. (2006). Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Research, 66, 9054–9064.
Ben-Shoshan, J., Maysel-Auslender, S., Mor, A., Keren, G., & George, J. (2008). Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. European Journal of Immunology, 38, 2412–2418.
Dayan, F., Mazure, N. M., Brahimi-Horn, M. C., & Pouyssegur, J. (2008). A dialogue between the hypoxia-inducible factor and the tumor microenvironment. Cancer Microenviron, 1, 53–68.
Sitkovsky, M. V., Kjaergaard, J., Lukashev, D., & Ohta, A. (2008). Hypoxia-adenosinergic immunosuppression: Tumor protection by T regulatory cells and cancerous tissue hypoxia. Clinical Cancer Research, 14, 5947–5952.
Harizi, H., Juzan, M., Pitard, V., Moreau, J. F., & Gualde, N. (2002). Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. Journal of Immunology, 168, 2255–2263.
Baratelli, F., Lin, Y., Zhu, L., Yang, S. C., Heuze-Vourc’h, N., Zeng, G., et al. (2005). Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. Journal of Immunology, 175, 1483–1490.
Akasaki, Y., Liu, G., Chung, N. H., Ehtesham, M., Black, K. L., & Yu, J. S. (2004). Induction of a CD4+ T regulatory type 1 response by cyclooxygenase-2-overexpressing glioma. Journal of Immunology, 173, 4352–4359.
Bergmann, C., Strauss, L., Zeidler, R., Lang, S., & Whiteside, T. L. (2007). Expansion of human T regulatory type 1 cells in the microenvironment of cyclooxygenase 2 overexpressing head and neck squamous cell carcinoma. Cancer Research, 67, 8865–8873.
Rodriguez, P. C., Hernandez, C. P., Quiceno, D., Dubinett, S. M., Zabaleta, J., Ochoa, J. B., et al. (2005). Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. The Journal of Experimental Medicine, 202, 931–939.
Murakami, A., & Ohigashi, H. (2007). Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. International Journal of Cancer, 121, 2357–2363.
Gately, S., & Li, W. W. (2004). Multiple roles of COX-2 in tumor angiogenesis: A target for antiangiogenic therapy. Seminars in Oncology, 31, 2–11.
Sharma, S., Yang, S. C., Zhu, L., Reckamp, K., Gardner, B., Baratelli, F., et al. (2005). Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Research, 65, 5211–5220.
Haas, A. R., Sun, J., Vachani, A., Wallace, A. F., Silverberg, M., Kapoor, V., et al. (2006). Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clinical Cancer Research, 12, 214–222.
Greenhough, A., Smartt, H. J., Moore, A. E., Roberts, H. R., Williams, A. C., Paraskeva, C., et al. (2009). The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis, 30, 377–386.
Motzer, R. J., Michaelson, M. D., Redman, B. G., Hudes, G. R., Wilding, G., Figlin, R. A., et al. (2006). Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. Journal of Clinical Oncology, 24, 16–24.
Norden-Zfoni, A., Desai, J., Manola, J., Beaudry, P., Force, J., Maki, R., et al. (2007). Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clinical Cancer Research, 13, 2643–2650.
Dellapasqua, S., Bertolini, F., Bagnardi, V., Campagnoli, E., Scarano, E., Torrisi, R., et al. (2008). Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. Journal of Clinical Oncology, 26, 4899–4905.
Bertolini, F., Shaked, Y., Mancuso, P., & Kerbel, R. S. (2006). The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nature Reviews. Cancer, 6, 835–845.
Farace, F., Massard, C., Borghi, E., Bidart, J. M., & Soria, J. C. (2007). Vascular disrupting therapy-induced mobilization of circulating endothelial progenitor cells. Annals of Oncology, 18, 1421–1422.
Ebos, J. M., Lee, C. R., Christensen, J. G., Mutsaers, A. J., & Kerbel, R. S. (2007). Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proceedings of the National Academy of Sciences of the United States of America, 104, 17069–17074.
Brown, A. P., Citrin, D. E., & Camphausen, K. A. (2008). Clinical biomarkers of angiogenesis inhibition. Cancer and Metastasis Reviews, 27, 415–434.
Rini, B. I., Michaelson, M. D., Rosenberg, J. E., Bukowski, R. M., Sosman, J. A., Stadler, W. M., et al. (2008). Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. Journal of Clinical Oncology, 26, 3743–3748.
Prior, J. O., Montemurro, M., Orcurto, M. V., Michielin, O., Luthi, F., Benhattar, J., et al. (2009). Early prediction of response to sunitinib after imatinib failure by 18F-fluorodeoxyglucose positron emission tomography in patients with gastrointestinal stromal tumor. Journal of Clinical Oncology, 27, 439–445.
Michael, A., Relph, K., & Pandha, H. (2010). Emergence of potential biomarkers of response to anti-angiogenic anti-tumour agents. International Journal of Cancer, 127, 1251–1258.
Lamuraglia, M., Escudier, B., Chami, L., Schwartz, B., Leclere, J., Roche, A., et al. (2006). To predict progression-free survival and overall survival in metastatic renal cancer treated with sorafenib: Pilot study using dynamic contrast-enhanced Doppler ultrasound. European Journal of Cancer, 42, 2472–2479.
Fournier, L. S., Oudard, S., Thiam, R., Trinquart, L., Banu, E., Medioni, J., et al. (2010). Metastatic renal carcinoma: Evaluation of antiangiogenic therapy with dynamic contrast-enhanced CT. Radiology, 256, 511–518.
Thiam, R., Fournier, L. S., Trinquart, L., Medioni, J., Chatellier, G., Balvay, D., et al. (2010). Optimizing the size variation threshold for the CT evaluation of response in metastatic renal cell carcinoma treated with sunitinib. Annals of Oncology, 21, 936–941.
Escudier, B., Pluzanska, A., Koralewski, P., Ravaud, A., Bracarda, S., Szczylik, C., et al. (2007). Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet, 370, 2103–2111.
Rixe, O., Bukowski, R. M., Michaelson, M. D., Wilding, G., Hudes, G. R., Bolte, O., et al. (2007). Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: A phase II study. The Lancet Oncology, 8, 975–984.
Yang, J. C., Haworth, L., Sherry, R. M., Hwu, P., Schwartzentruber, D. J., Topalian, S. L., et al. (2003). A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. The New England Journal of Medicine, 349, 427–434.
Faivre, S., Delbaldo, C., Vera, K., Robert, C., Lozahic, S., Lassau, N., et al. (2006). Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. Journal of Clinical Oncology, 24, 25–35.
van Cruijsen, H., van der Veldt, A. A., Vroling, L., Oosterhoff, D., Broxterman, H. J., Scheper, R. J., et al. (2008). Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clinical Cancer Research, 14, 5884–5892.
George, S., Richmond, A., Elson, P., Jin, T., Wood, L., Garcia, J. A., et al. (2007). WBC changes as a pharmacodynamic marker of outcome in metastatic renal cell carcinoma (mRCC) patients (Pts) receiving sunitinib (p. 5043). Chicago: ASCO.
Griffiths, R. W., Elkord, E., Gilham, D. E., Ramani, V., Clarke, N., Stern, P. L., et al. (2007). Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunology, Immunotherapy, 56, 1743–1753.
Shojaei, F., Wu, X., Malik, A. K., Zhong, C., Baldwin, M. E., Schanz, S., et al. (2007). Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature Biotechnology, 25, 911–920.
Huang, D., Ding, Y., Zhou, M., Rini, B. I., Petillo, D., Qian, C. N., et al. (2010). Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Research, 70, 1063–1071.
Nikolinakos, P. G., Altorki, N., Yankelevitz, D., Tran, H. T., Yan, S., Rajagopalan, D., et al. (2010). Plasma cytokine and angiogenic factor profiling identifies markers associated with tumor shrinkage in early-stage non-small cell lung cancer patients treated with pazopanib. Cancer Research, 70, 2171–2179.
Hanrahan, E. O., Lin, H. Y., Kim, E. S., Yan, S., Du, D. Z., McKee, K. S., et al. (2010). Distinct patterns of cytokine and angiogenic factor modulation and markers of benefit for vandetanib and/or chemotherapy in patients with non-small-cell lung cancer. Journal of Clinical Oncology, 28, 193–201.
Tartour, E., Mosseri, V., Jouffroy, T., Deneux, L., Jaulerry, C., Brunin, F., et al. (2001). Serum soluble interleukin-2 receptor concentrations as an independent prognostic marker in head and neck cancer. Lancet, 357, 1263–1264.
Tartour, E., Deneux, L., Mosseri, V., Jaulerry, C., Brunin, F., Point, D., et al. (1997). Soluble interleukin-2 receptor serum level as a predictor of locoregional control and survival for patients with head and neck carcinoma: Results of a multivariate prospective study. Cancer, 79, 1401–1408.
Saltz, L. B., Rosen, L. S., Marshall, J. L., Belt, R. J., Hurwitz, H. I., Eckhardt, S. G., et al. (2007). Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. Journal of Clinical Oncology, 25, 4793–4799.
Bergers, G., & Hanahan, D. (2008). Modes of resistance to anti-angiogenic therapy. Nature Reviews. Cancer, 8, 592–603.
Willett, C. G., Boucher, Y., di Tomaso, E., Duda, D. G., Munn, L. L., Tong, R. T., et al. (2004). Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Natural Medicines, 10, 145–147.
Hamzah, J., Jugold, M., Kiessling, F., Rigby, P., Manzur, M., Marti, H. H., et al. (2008). Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature, 453, 410–414.
Dirkx, A. E., oude Egbrink, M. G., Castermans, K., van der Schaft, D. W., Thijssen, V. L., Dings, R. P., et al. (2006). Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB Journal, 20, 621–630.
Li, B., Lalani, A. S., Harding, T. C., Luan, B., Koprivnikar, K., Huan Tu, G., et al. (2006). Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clinical Cancer Research, 12, 6808–6816.
Manning, E. A., Ullman, J. G., Leatherman, J. M., Asquith, J. M., Hansen, T. R., Armstrong, T. D., et al. (2007). A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clinical Cancer Research, 13, 3951–3959.
Shrimali, R. K., Yu, Z., Theoret, M. R., Chinnasamy, D., Restifo, N. P., & Rosenberg, S. A. (2010). Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Research, 70, 6171–6180.
Nair, S., Boczkowski, D., Moeller, B., Dewhirst, M., Vieweg, J., & Gilboa, E. (2003). Synergy between tumor immunotherapy and antiangiogenic therapy. Blood, 102, 964–971.
Bercovici, N., Haicheur, N., Massicard, S., Vernel-Pauillac, F., Adotevi, O., Landais, D., et al. (2008). Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. Journal of Immunotherapy, 31, 101–112.
Huang, K. W., Wu, H. L., Lin, H. L., Liang, P. C., Chen, P. J., Chen, S. H., et al. (2010). Combining antiangiogenic therapy with immunotherapy exerts better therapeutical effects on large tumors in a woodchuck hepatoma model. Proceedings of the National Academy of Sciences of the United States of America, 107, 14769–14774.
Kerzerho, J., Adotevi, O., Castelli, F. A., Dosset, M., Bernardeau, K., Szely, N., et al. (2010). The angiogenic growth factor and biomarker midkine is a tumor-shared antigen. Journal of Immunology, 185, 418–423.
Molhoek, K. R., McSkimming, C. C., Olson, W. C., Brautigan, D. L., & Slingluff, C. L., Jr. (2009). Apoptosis of CD4(+)CD25(high) T cells in response to Sirolimus requires activation of T cell receptor and is modulated by IL-2. Cancer Immunology, Immunotherapy, 58, 867–876.
Albini, A., Brigati, C., Ventura, A., Lorusso, G., Pinter, M., Morini, M., et al. (2009). Angiostatin anti-angiogenesis requires IL-12: The innate immune system as a key target. Journal of Translational Medicine, 7, 5.
Rini, B. I., Halabi, S., Rosenberg, J. E., Stadler, W. M., Vaena, D. A., Ou, S. S., et al. (2008). Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. Journal of Clinical Oncology, 26, 5422–5428.
Flaherty, K. T. (2010). Where does the combination of sorafenib and interferon in renal cell carcinoma stand? Cancer, 116, 4–7.
Zhao, W., Gu, Y. H., Song, R., Qu, B. Q., & Xu, Q. (2008). Sorafenib inhibits activation of human peripheral blood T cells by targeting LCK phosphorylation. Leukemia, 22, 1226–1233.
Houben, R., Voigt, H., Noelke, C., Hofmeister, V., Becker, J. C., & Schrama, D. (2009). MAPK-independent impairment of T-cell responses by the multikinase inhibitor sorafenib. Molecular Cancer Therapeutics, 8, 433–440.
Acknowledgments
This work was supported by Canceropole Ile de France, ANR (Agence Nationale de la Recherche), Ligue contre le Cancer, Association pour la Recherche sur le Cancer, Pole de compétitivité Medicen (Immucan), Centre d’investigation Clinique en Biothérapie (CIC-BT). AP-HP. INSERM, Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tartour, E., Pere, H., Maillere, B. et al. Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30, 83–95 (2011). https://doi.org/10.1007/s10555-011-9281-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9281-4