Abstract
Purpose
Tobacco smoke could cause childhood acute lymphoblastic leukemia (ALL) through at least three pathways: (1) prenatal parental smoking; (2) fetal exposure through maternal smoking during pregnancy; and (3) childhood exposure to secondhand smoke (SHS). We tested these hypotheses in a large population-based case–control study (SETIL) primarily designed to evaluate the role of electromagnetic fields in childhood hematopoietic malignancies.
Methods
From 1998 to 2003, we enrolled 602 incident cases of ALL from 14 Italian Regions, and 918 controls were individually matched by birthdate, sex, and area of residence. Cases (n = 557) and controls (n = 855) with complete information were analyzed; odds ratios (OR) and 95 % confidence intervals (95 % CI) were estimated with logistic regression models conditioned on matching variables and adjusted by birth order, birthweight, duration of breastfeeding, parental age at delivery, education, and occupational exposure to benzene.
Results
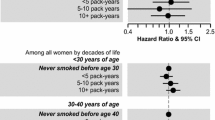
No evidence associating paternal smoking in the conception period or maternal smoking during the pregnancy with ALL was found. An association of ALL with maternal exposure to SHS during pregnancy (adjusted OR for mothers exposed more than 4 h/day = 2.18, 95 % CI 1.39–3.42) was observed, but recall bias cannot be excluded. Exposure of the children to SHS was associated with ALL only in unadjusted analysis (unadjusted OR for highly exposed children = 1.64; 95 % CI 1.10–2.45).
Conclusions
This study does not support the hypothesis that parental active smoking is associated with ALL. We found very weak evidence of increased risk of ALL for children exposed to SHS. Maternal exposure to SHS was associated with ALL, but recall bias is likely to inflate our estimates.


Similar content being viewed by others
References
Greaves MF, Alexander FE (1993) An infectious etiology for common acute lymphoblastic leukemia in childhood? Leukemia 7:349–360
Belson M, Kingsley B, Holmes A (2007) Risk factors for acute leukemia in children: a review. Environ Health Perspect 115:138–145
Eden T (2010) Aetiology of childhood leukaemia. Cancer Treat Rev 36:286–297
Greaves M (2006) Infection, immune responses and the aetiology of childhood leukaemia. Nat Rev Cancer 6:193–203
WHO-IARC (2009) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 100. A Review of Human Carcinogens. Part E: Personal Habits and Indoor Combustions. WHO Press, Lyon
Greaves M (2005) In utero origins of childhood leukaemia. Early Hum Dev 81:123–129
Chang JS (2009) Parental smoking and childhood leukemia. Methods Mol Biol 472:103–137
Lee KM, Ward MH, Han S, Ahn HS, Kang HJ, Choi HS et al (2009) Paternal smoking, genetic polymorphisms in CYP1A1 and childhood leukemia risk. Leuk Res 33:250–258
Milne E, Greenop KR, Scott RJ, Bailey HD, Attia J, Dalla-Pozza L et al (2012) Parental prenatal smoking and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol 175:43–53
Mucci LA, Granath F, Cnattingius S (2004) Maternal smoking and childhood leukemia and lymphoma risk among 1,440,542 Swedish children. Cancer Epidemiol Biomarkers Prev 13:1528–1533
Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, Al-Aidrous F, De Hoogh K, Magnani C, Forastiere F; on behalf of the SETIL Study Group (2013) Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup Environ Med. doi:10.1136/oemed-2013-101604
Miligi L, Benvenuti A, Mattioli S, Salvan A, Tozzi GA, Ranucci A et al (2013) Risk of childhood leukaemia and non-Hodgkin’s lymphoma after parental occupational exposure to solvents and other agents: the SETIL Study. Occup Environ Med 70:648–655
AIRTUM Working Group (2008) Italian cancer figures-report 2008. 1. Childhood cancer. (In Italian) Epidemiol Prev 32:1, 5-13, 16-35
Breslow NE, Day NE (1980) Statistical Methods in Cancer Research, vol 1. International Agency for Research on Cancer, Lyon
Trédaniel J, Boffetta P, Little J, Saracci R, Hirsch A (1994) Exposure to passive smoking during pregnancy and childhood, and cancer risk: the epidemiological evidence. Paediatr Perinat Epidemiol 8:233–255
Sasco AJ, Vainio H (1999) From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol 18:192–201
Crawford FG, Mayer J, Santella RM, Cooper TB, Ottman R, Tsai WY et al (1994) Biomarkers of environmental tobacco smoke in preschool children and their mothers. J Natl Cancer Inst 86:1398–1402
Tang D, Warburton D, Tannenbaum SR, Skipper P, Santella RM, Cereijido GS et al (1999) Molecular and genetic damage from environmental tobacco smoke in young children. Cancer Epidemiol Biomarkers Prev 8:427–431
Law MR, Hackshaw AK (1996) Environmental tobacco smoke. Br Med Bull 52:22–34
U.S. EPA (1992) Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. EPA/600/6-90/006F. U.S. Environmental Protection Agency, Office of Research and Development, Washington
Boffetta P, Trédaniel J, Greco A (2000) Risk of childhood cancer and adult lung cancer after childhood exposure to passive smoke: a meta-analysis. Environ Health Perspect 108:73–82
Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN (1996) Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutat Res 351:199–203
Shen HM, Chia SE, Ni ZY, New AL, Lee BL, Ong CN (1997) Detection of oxidative DNA damage in human sperm and the association with cigarette smoking. Reprod Toxicol 11:675–680
Shi Q, Ko E, Barclay L, Hoang T, Rademaker A, Martin R (2001) Cigarette smoking and aneuploidy in human sperm. Mol Reprod Dev 59:417–421
Zenzes MT, Bielecki R, Reed TE (1999) Detection of benzo(a)pyrene diol epoxide-DNA adducts in sperm of men exposed to cigarette smoke. Fertil Steril 72:330–335
Zenzes MT, Puy LA, Bielecki R, Reed TE (1999) Detection of benzo(a)pyrene diol epoxide-DNA adducts in embryos from smoking couples: evidence for transmission by spermatozoa. Mol Hum Reprod 5:125–131
Liu R, Zhang L, McHale CM, Hammond SK (2011) Paternal smoking and risk of childhood acute lymphoblastic leukemia: systematic review and meta-analysis. J Oncol. doi:10.1155/2011/854584
Kelsey JL, Whittemore AS, Evans AS, Thompson WD (1996) Methods in observational epidemiology. Oxford University Press, New York
Ammenheuser MM, Berenson AB, Stiglich NJ, Whorton EB, Ward JB (1994) Elevated frequencies of hprt mutant lymphocytes in cigarette-smoking mothers and their newborns. Mutat Res 304:285–294
de la Chica RA, Ribas I, Giraldo J, Egozcue J, Fuster C (2005) Chromosomal instability in amniocytes from fetuses of mothers who smoke. JAMA 293:1212–1222
Klimentopoulou A, Antonopoulos CN, Papadopoulou C, Kanavidis P, Tourvas AD, Polychronopoulou S et al (2012) Maternal smoking during pregnancy and risk for childhood leukemia: a nationwide case-control study in Greece and meta-analysis. Pediatr Blood Cancer 58:344–351
Ng SP, Silverstone AE, Lai ZW, Zelikoff JT (2006) Effects of prenatal exposure to cigarette smoke on offspring tumor susceptibility and associated immune mechanisms. Toxicol Sci 89:135–144
Cnattingius S (2004) The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res 6:S125–S140
Yeazel MW, Buckley JD, Woods WG, Ruccione K, Robison LL (1995) History of maternal fetal loss and increased risk of childhood acute leukaemia at an early age. A report from the Childrens Cancer Group. Cancer 75:1718–1727
Perrillat F, Clavel J, Jaussent I, Baruchel A, Leverger G, Nelken B et al (2002) Breast-feeding, fetal loss and childhood acute leukaemia. Eur J Pediatr 161:235–237
Chang JS, Selvin S, Metayer C, Crouse V, Golembesky A, Buffler PA (2006) Parental smoking and the risk of childhood leukemia. Am J Epidemiol 163:1091–1100
ISTAT (2005) Stili di vita e condizioni di salute. Indagine multiscopo sulle famiglie “Aspetti della vita quotidiana”. Anno 2003. Istituto nazionale di statistica, Roma
EpiCentro (2013) I dati sul fumo. www.epicentro.iss.it/temi/fumo/dati.asp. Accessed 16 January 2014
Post A, Gilljam H, Bremberg S, Galanti MR (2008) Maternal smoking during pregnancy: a comparison between concurrent and retrospective self-reports. Paediatr Perinat Epidemiol 22:155–161
Acknowledgments
The SETIL study was financially supported by research grants received by AIRC (Italian Association on Research on Cancer), MIUR (Ministry for Instruction, University and Research, PRIN Program), Ministry of Health (Ricerca Sanitaria Finalizzata Program), Ministry of Labour and Welfare, Associazione Neuroblastoma, Piemonte Region (Ricerca Sanitaria Finalizzata Regione Piemonte Program), Liguria Region, Comitato per la vita “Daniele Chianelli”-Associazione per la Ricerca e la Cura delle Leucemie, Linfomi e Tumori di Adulti e Bambini (Perugia). Andrea Farioli’s work on this paper was supported by the Master in Epidemiology, University of Turin. We would like to acknowledge the contributors to the design and conduct of the SETIL study: Antonio Acquaviva, AOU di Siena, Siena, Italia; Daniele Andreuccetti, I.R.O.E.—CNR—Firenze, Italia; Laura Anglesio, ARPA—Ivrea (To), Italia; Maurizio Aricò, AOU Meyer, Firenze, Italia; Giorgio Assennato, ARPA—Puglia, Bari, Italia; Francesco Barone Adesi, CPO Piemonte, Torino, Italia; Isabella Belletti, National Cancer Institute, Milano, Italia; Gabriella Bernini, AOU Meyer, Firenze, Italia; Marinella Bertolotti, CPO Piemonte, Torino, Italia; Paolo Bevitori, ARPA Rimini, Rimini, Italia; Renzo Biancotto, ARPAV—Venezia, Italia; Pierfranco Biddau, Ospedale Microcitemico, Cagliari, Italia; Annibale Biggeri, Università degli Studi di Firenze, Firenze, Italia; Luigi Bisanti, ASL di Milano, Milano, Italia; Vittorio Bocchini, Istituto Nazionale per la Ricerca sul Cancro, Genova, Italia; Francesco Bochicchio, Istituto Superiore di Sanità, Roma, Italia; Silvia Bucci, ARPAT, Firenze, Italia; Roberto Calisti, SPreSAL, Civitanova Marche, Italia; Santina Cannizzaro, Lega Italiana per la Lotta contro i Tumori Onlus, Ragusa, Italia; Nicola Caranci, Agenzia sanitaria e sociale regionale, Emilia-Romagna, Bologna, Italia; Veronica Casotto, IRCCS Burlo Garofolo, Trieste, Italia; Fulvio Cavariani, Centro Regionale Amianto, Viterbo, Italia; Egidio Celentano, ARSAN—Agenzia Regionale Sanitaria della Campania, Napoli, Italia; Manuela Chiavarini, Università degli Studi di Perugia, Perugia, Italia; Pierluigi Cocco, Università di Cagliari, Cagliari, Italia; Pietro Comba, Istituto Superiore di Sanità, Roma, Italia; Paolo Crosignani, Istituto Nazionale Tumori, Milano, Italia; Marina Cuttini, Ospedale Pediatrico Bambino Gesù, Roma, Italia; Giovanni d’Amore, ARPA, Ivrea (To), Italia; Gigliola de Nichilo, SPESAL, Barletta, Italia; Gian Luca De Salvo, Istituto Oncologico Veneto IRCCS, Padova, Italia; Elena Duglio, IRCCS AOU San Martino-ITS, IST Genova, Italia; Myris Erna, PMP Fisica, Padova, Italia; Daniela Ferrante, CPO Piemonte, Novara, Italia; Francesco Forastiere, Dipartimento Epidemiologia Regione Lazio, Roma, Italia; Lorenzo Gafà, Lega Italiana per la Lotta contro i Tumori Onlus, Ragusa, Italia; Claudia Galassi, AOU S.Giovanni Battista e CPO Piemonte, Torino, Italia; Luigi Gelli, Struttura Regionale dell’Autorità Ambientale, Regione Campania, Napoli, Italia; Marco Gilardetti, CPO Piemonte, Torino, Italia; Erni Guarino, Istituto Nazionale Tumori, Napoli, Italia; Paolo Guidotti, ITI, Firenze, Italia; Riccardo Haupt, Istituto Giannina Gaslini, Genova, Italia; Ursula Kirchmayer, Dipartimento Epidemiologia Regione Lazio, Roma, Italia; Susanna Lagorio, National Institute of Health, Rome, Italia; Alma Lippi, AOU Meyer, Firenze, Italia; Franco Locatelli, Università di Pavia, Pavia, Italia; Mirti Lombardi, PMP, Ancona, Italia; Dana Loomis, University of North Carolina—USA; Lia Lidia Luzzatto, ASL 1—Torino, Torino, Italia; Mauro Magnoni, ARPA—Ivrea (To), Italia; Giuseppe Masera, Università Milano Bicocca, Monza, Italia; Pia Massaglia, Neuropsichiatria Infantile, Torino, Italia; Franco Merletti, Università di Torino, Torino, Italia; Domenico Franco Merlo, IRCCS Azienda Ospedaliera Universitaria San Martino- IST Istituto Nazionale per la Ricerca sul Cancro, Genova, Italia; Giuseppe Miceli, Azienda Sanitaria Locale.7, Ragusa, Italia; Paola Michelozzi, Dipartimento Epidemiologia Regione Lazio, Roma, Italia; Liliana Minelli, Università degli Studi di Perugia, Perugia, Italia; Daniele Monetti, Istituto Oncologico Veneto IRCCS, Padova, Italia; Paola Mosciatti, Università di Camerino, Camerino, Italia; Alessandra Greco, Istituto Oncologico Veneto IRCCS, Padova, Italia; Piero Mozzo, Centro Radioattività Ambientale, Verona, Italia; Margherita Nardi, AOU di Pisa, Pisa, Italia; Cristina Nuccetelli, Istituto Superiore di Sanità, Roma, Italia; Salvatore Panico, Università degli Studi di Napoli, Napoli, Italia; Franco Pannelli, Registro Tumori di Macerata e Università di Camerino, Camerino, Italia; Guido Paolucci, University of Bologna, Bologna, Italia; Andrea Pession, University of Bologna, Bologna, Italia; Alessandro Polichetti, National Institute of Health, Roma, Italia; Andrea Poggi, ARPAT, Firenze, Italia; Vincenzo Poggi, A.O.R.N. Santobono—Pausilipon, Napoli, Italia; Ombretta Pons, Dip. Scienze Biomediche, Torino, Italia; Alessandro Pulsoni, La Sapienza, Università di Roma, Roma, Italia; Assunta Rasulo, CPO Piemonte, Torino, Italia; Serena Risica, Istituto Superiore di Sanità, Roma, Italia; Carmelo Rizzari, A.O. San Gerardo, Fondazione MBBM, Monza, Italia; Stefano Roletti, ARPA, Ivrea (To), Italia; Maria Rosa, Sez.Fisica Ambientale, Mestre (Ve), Italia; Ornella Ru, CPO Piemonte, Torino, Italia; Giovanna Russo, Università di Catania, Catania, Italia; Giuseppe Sampietro, ASL Città di Milano, Milano, Italia; Gino Schilirò, Università di Catania, Catania, Italia; Giuseppe Sgorbati, PMP, Milano, Italia; Stefano Silvestri, ISPO, Firenze, Italia; Lorenzo Simonato, Università degli Studi di Padova, Padova, Italia; Donato Sivo, Università di Bari, Bari, Italia; Letizia Sommani, ASF Firenze, Firenze, Italia; Bianca Stievano, Sez.Fisica Ambientale, Padova, Italia; Benedetto Terracini, Dip. Scienze Biomediche, Torino, Italia; Santi Tofani, ASL Ivrea, Ivrea, Italia; Maria Valeria Torregrossa, Università degli Studi di Palermo; Palermo, Italia; Loredana Troeschel, National Cancer Institute, Milano, Italia; Flavio Troti, Centro Radioattività Ambientale, Verona, Italia; Rosario Tumino, Registro Tumori di Ragusa, Ragusa, Italia; Rosaria Maria Valenti, Università degli Studi di Palermo, Palermo, Italia; Massimo Valle, PMP Fisica, Genova, Italia; Stefania Varotto, Dipartimento di Pediatria, Università di Padova, Italia; Carmen Fiorella Stocco, Registro Tumori del Veneto, Istituto Oncologico Veneto IRCCS, Padova, Italia; Paolo Vecchia, ISS, Roma, Italia; Paola Zambon, Registro Tumori del Veneto, Università di Padova, Padova, Italia.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the SETIL Study Group.
Patrizia Legittimo: previously at Occupational and Environmental Epidemiology Unit, ISPO Cancer Prevention and Research Institute, Florence, Italy.
Alberto Salvan: retired.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farioli, A., Legittimo, P., Mattioli, S. et al. Tobacco smoke and risk of childhood acute lymphoblastic leukemia: findings from the SETIL case–control study. Cancer Causes Control 25, 683–692 (2014). https://doi.org/10.1007/s10552-014-0371-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-014-0371-9