Abstract
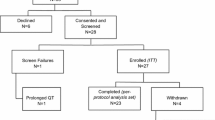
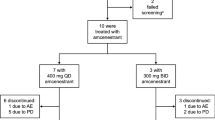
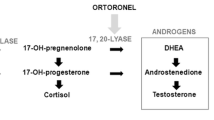
Steroid sulfatase (STS) inhibition may have a therapeutic role in suppression of endocrine-responsive breast cancer. This study aimed to determine the optimal biological dose and recommended dose (RD) of the STS inhibitor irosustat. A three-part, open-label, multicenter, dose escalation study of irosustat in estrogen receptor-positive breast cancer patients involved administration of a single dose of irosustat with a 7-day observation period; followed by a daily oral dose of irosustat for 28 days; and an extension phase, in which the daily oral dose of irosustat was continued at the discretion of the investigator and as long as the patient was benefitting from the treatment. Five doses of irosustat were tested (1, 5, 20, 40, and 80 mg) in 50 patients. After 28 days of daily administration of irosustat, all the evaluated patients in the 5, 20, 40, and 80 mg cohorts achieved ≥95 % STS inhibition in peripheral blood mononuclear cells and corresponding endocrine suppression. The maximum tolerated dose was not reached, and the 40 mg dose was established as the RD. The median time to disease progression in the 40 mg cohort was 11.2 weeks. Disease stabilization was achieved in 10 % of patients potentially indicative of drug activity. Dry skin was the most frequent adverse event. The RD of irosustat is 40 mg. Disease stabilization occurred in 10 % of this heavily pretreated patient population. A larger study is required to define an accurate response rate to irosustat as a single agent and whether co-administration with an aromatase inhibitor is needed.



Similar content being viewed by others
References
James VH, McNeill JM, Lai LC, Newton CJ, Ghilchik MW, Reed MJ (1987) Aromatase activity in normal breast and breast tumor tissues: in vivo and in vitro studies. Steroids 50(1–3):269–279
Santner SJ, Feil PD, Santen RJ (1984) In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab 59(1):29–33
Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H (2003) Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res 63(11):2762–2770
Utsumi T, Yoshimura N, Takeuchi S, Ando J, Maruta M, Maeda K, Harada N (1999) Steroid sulfatase expression is an independent predictor of recurrence in human breast cancer. Cancer Res 59(2):377–381
Miyoshi Y, Ando A, Hasegawa S, Ishitobi M, Taguchi T, Tamaki Y, Noguchi S (2003) High expression of steroid sulfatase mRNA predicts poor prognosis in patients with estrogen receptor-positive breast cancer. Clin Cancer Res 9(6):2288–2293
Yoshimura N, Harada N, Bukholm I, Karesen R, Borresen-Dale AL, Kristensen VN (2004) Intratumoural mRNA expression of genes from the oestradiol metabolic pathway and clinical and histopathological parameters of breast cancer. Breast Cancer Res 6(2):R46–R55. doi:10.1186/bcr746
Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, Wilson RH, Stanczyk FZ, Dobbs N, Kulinskaya E, Elliott M, Potter BV, Reed MJ, Coombes RC (2006) Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res 12(5):1585–1592. doi:10.1158/1078-0432.CCR-05-1996
Chanplakorn N, Chanplakorn P, Suzuki T, Ono K, Chan MS, Miki Y, Saji S, Ueno T, Toi M, Sasano H (2010) Increased estrogen sulfatase (STS) and 17beta-hydroxysteroid dehydrogenase type 1(17beta-HSD1) following neoadjuvant aromatase inhibitor therapy in breast cancer patients. Breast Cancer Res Treat 120(3):639–648. doi:10.1007/s10549-010-0785-3
National Institute of Health National Cancer Institute. Common criteria for adverse events v3.0 (CTCAE) (2006). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 4 April 2013
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M (2008) Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol 26(10):1664–1670. doi:10.1200/JCO.2007.13.5822
Bruning PF, Bonfrer JM, Paridaens R, Nooij M, Klijn JG, Beex LV, Bruynseels J, Piccart MJ (1998) Vorozole (R83842) in the treatment of postmenopausal advanced breast cancer: relationship of serum levels of vorozole and clinical results (a study of the EORTC Breast Cancer Cooperative Group). Anticancer Drugs 9(5):419–425
Buzdar AU, Jonat W, Howell A, Jones SE, Blomqvist CP, Vogel CL, Eiermann W, Wolter JM, Steinberg M, Webster A, Lee D (1998) Anastrozole versus megestrol acetate in the treatment of postmenopausal women with advanced breast carcinoma: results of a survival update based on a combined analysis of data from two mature phase III trials Arimidex Study Group. Cancer 83(6):1142–1152. doi:10.1002/(SICI)1097-0142(19980915)83:6<1142:AID-CNCR13>3.0.CO;2-5
Ventura V, Sola J, Celma C, Peraire C, Obach R (2011) In vitro metabolism of irosustat, a novel steroid sulfatase inhibitor: interspecies comparison, metabolite identification, and metabolic enzyme identification. Drug Metab Dispos 39(7):1235–1246. doi:10.1124/dmd.111.038315
Acknowledgments
The study was funded by Ipsen. Kymos Pharma Services (Barcelona, Spain) provided hormone analysis, Beckman Coulter Genomics (formerly Epidauros Biotechnologie AG), Bernried, Germany provided pharmacogenetic analyses, Pharmacokinetics Department at Ipsen Pharma, Spain conducted PK plasma level analyses and PK/PD analyses, and the Drug Metabolism Research Laboratory at Institut Biologie et de Technologies de Saclay (LEMM; Gif-Sur-Yvette, France) did STS activity analyses. 18F-FDG-PET analysis was performed by Pr Daniel Slosman of Qualim (Genève Switzerland). Editorial support for this article was provided by Martin Gilmour of ESP Bioscience (Crowthorne, UK), funded by Ipsen. We thank the Imperial BRC and ECMC for support, as well as Cancer Research UK.
Conflict of interest
RCC, FC, NI, PSchmid and PSoulie declare that they have no conflict of interest. TL was consultant or advisor (compensated) for Roche and provided expert testimony (compensated) for Bristol-Myers Squibb within the last 3 years, and was also a consultant/advisor for GSK. CP and AK were employees of Ipsen Pharma S.A. VF was employee of Ipsen Innovation S.A. TA was an employee of Ipsen Biopharm Ltd.
Ethical standards
The trial was conducted according to the Declaration of Helsinki and International Conference on Harmonization of Good Clinical Practice. All applicable regulatory requirements, and local independent ethics committee and institutional review board approvals were obtained before starting the trial. All patients gave written informed consent to participate in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coombes, R.C., Cardoso, F., Isambert, N. et al. A phase I dose escalation study to determine the optimal biological dose of irosustat, an oral steroid sulfatase inhibitor, in postmenopausal women with estrogen receptor-positive breast cancer. Breast Cancer Res Treat 140, 73–82 (2013). https://doi.org/10.1007/s10549-013-2597-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-013-2597-8