Abstract
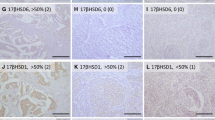
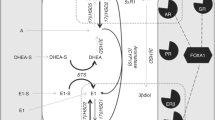
Aromatase inhibitors (AIs) are considered the gold standard for endocrine therapy of estrogen receptor (ER) positive postmenopausal breast cancer patients. The therapy may enhance therapeutic response and stabilize disease but resistance and disease progression inevitably occur in the patients. These are considered at least partly due to an emergence of alternative intratumoral estrogen production pathways. Therefore, in this study we evaluated effects of exemestane (EXE) upon the enzymes involved in intratumoral estrogen production including estrogen sulfatase (STS), 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1), and estrogen sulfotransferase (EST) and correlated the findings with therapeutic responses including Ki67 labeling index (Ki67). 116 postmenopausal patients with invasive ductal carcinoma, stage II/IIIa, were enrolled in JFMC34-0601 clinical trials between March, 2006 and January, 2008. EXE of 25 mg/day was administered according to the protocol. Pre- and posttreatment specimens of 49 cases were available for this study. Status of STS, EST, 17β-HSD1, ER, progesterone receptor (PgR), human epidermal growth factor receptor type 2 (Her2), and Ki67 in pre- and post-specimens were evaluated. Specimens examined before the therapy demonstrated following features; ER+ (100%), PgR+ (85.7%), and Her2+ (77.6%). After treatment, the number of Ki67, PgR, and ER positive carcinoma cells demonstrated significant decrement in clinical response (CliR) and pathological response (PaR) groups. Significant increment of 17β-HSD1 and STS immunoreactivity was detected in all groups examined except for STS in PaR. EST showed significant increment in nonresponsive groups. Alterations of Ki67 of carcinoma cells before and after therapy were subclassified into three groups according to its degrees. Significant alterations of intratumoral enzymes, especially 17β-HSD1 and STS, were correlated with Ki67 reduction after neoadjuvant EXE therapy. This is the first study demonstrating significant increment of STS and 17β-HSD1 following AI neoadjuvant therapy of postmenopausal ER positive breast carcinoma patients. This increment may represent the compensatory response of breast carcinoma tissues to estrogen depletion.

Similar content being viewed by others
References
Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ (2007) Global Cancer Facts & Figures 2007. American Cancer Society, Atlanta, GA. http://www.cancer.org/docroot/STT/STT_0.asp. Accessed 19 Nov 2009
American Cancer Society (2009) Breast Cancer Facts & Figures 2009–2010. Atlanta, GA: American Cancer Society. http://www.cancer.org/docroot/STT/STT_0.asp. Accessed 19 Nov 2009
Pasqualini JR, Chetrite GS (2005) Recent insight on the control of enzymes involved in estrogen formation and transformation in human breast cancer. J Steroid Biochem Mol Biol 93:221–236
Chen S, Masri S, Hong YY, Wang X, Phung S, Yuan YC, Wu X (2007) New experimental models for aromatase inhibitor resistance. J Steroid Biochem Mol Biol 106:8–15. doi:10.1016/j.jsbmb.2007.05.020
Sasano H, Nagasaki S, Miki Y, Suzuki T (2009) New developments in intracrinology of human breast cancer estrogen sulfatase and sulfotransferase. Ann NY Acad Sci 1155:76–79
Miller WR, Forrest AP (1974) Oestradiol synthesis by a human breast carcinoma. Lancet 2:866–868
James VH, Reed MJ, Lai LC, Ghilchik MW, Tait GH, Newton CJ, Coldham NG (1990) Regulation of estrogen concentrations in human breast tissues. Ann NY Acad Sci 595:227–235
Larionov AA, Berstein LM, Miller WR (2002) Local uptake and synthesis of oestrone in normal and malignant postmenopausal breast tissues. J Steroid Biochem Mol Biol 81:57–64
Miller WR, Hawkins RA, Forrest APM (1982) Significance of aromatase activity in human breast cancer. Cancer Res 42:S3365–S3368
Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H (2007) Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res 67:3945–3954
Sasano H, Harada N (1998) Intratumoral aromatase in human breast, endometrial, and ovarian malignancies. Endocr Rev 19:593–607
Santen RJ, Leszczynski D, Tilson-Mallet N, Feil PD, Wright C, Manni A, Santner S (1986) Enzymatic control of estrogen production in human breast cancer: relative significance of aromatase versus sulfatase pathways. Ann N Y Acad Sci 464:126–137
Miller WR, Anderson TJ, Jack WJL (1990) Relationship between tumour aromatase activity, tumour characteristics and response to therapy. J Steroid Biochem Mol Biol 37:1055–1059
Suzuki T, Nakata T, Miki Y, Kaneko C, Moriya T, Ishida T, Akinaga S, Hirakawa H, Kimura M, Sasano H (2003) Estrogen sulfotransferase and steroid sulfatase in human breast carcinoma. Cancer Res 63:2762–2770
Sasano H, Suzuki T, Nakata T, Moriya T (2006) New development in intracrinology of breast carcinoma. Breast Cancer 13:129–136
Santen RJ (2003) Inhibition of aromatase: insights from recent studies. Steroids 68:559–567
Miller WR, Anderson TJ, White S, Larionov A, Murray J, Evans D, Krause A, Dixon JM (2005) Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol 95:83–89
Geisler J (2008) Aromatase inhibitors: from bench to bedside and back. Breast Cancer 15:17–26
Geisler J, Lønning PE (2005) Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res 11:2809–2821
Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S (2008) Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer Res 68:4910–4918
Lønning PE (2009) Lack of complete cross-resistance between different aromatase inhibitors; a real finding in search for an explanation? Eur J Cancer 45:527–535
Santen RJ, Lobenhofer EK, Afshari CA, Bao Y, Song RX (2005) Adaptation of estrogen-regulated genes in long-term estradiol deprived MCF-7 breast cancer cells. Breast Cancer Res Treat 94:213–223
Brodie A, Macedo L, Sabnis G (2009) Aromatase resistance mechanisms in model system in vivo. J Steroid Biochem Mol Biol. doi:10.1016/j.jsbmb.2009.09.004
Flågeng MH, Moi LL H, Dixon JM, Geisler J, Lien EA, Miller WR, LØnning PE, Mellgren G (2009) Nuclear receptor co-activators and HER-2/neu are upregulated in breast cancer patients during neo-adjuvant treatment with aromatase inhibitors. Br J Cancer 101:1253–1260
Sato N, Masuda N, Saji S, Takei H, Yamamoto Y, Sasano H, Toi M (2009) Neoadjuvant exemestane for 24 weeks in postmenopausal women with hormone receptor positive stage II or IIIa breast cancer (JFMC34-0601) [abstract 591]. J Clin Oncol 27:15S
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Miller WR, White S, Dixon JM, Murray J, Renshaw L, Anderson TJ (2006) Proliferation, steroid receptors and clinical/pathological response in breast cancer treated with letrozole. Br J Cancer 94:1051–1056
Bouzubar N, Walker KJ, Griffiths K, Ellis IO, Elston CW, Robertson JFR, Blamey RW, Nicholson RI (1989) Ki67 immunostaining in primary breast cancer: pathological and clinical associations. Br J Cancer 59:943–947
Harvey JM, Clark GM, Osborne CK, Allred DC (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol 17:1474–1481
Wolff AC, Hammond ME, Schwartz JN et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Takei H, Suemasu K, Inoue K, Saito T, Okubo K, Koh J, Sato K, Tsuda H, Kurosumi M, Tabei T (2008) Multicenter phase II trial of neoadjuvant exemestane for postmenopausal patients with hormone receptor-positive, operable breast cancer: Saitama Breast Cancer Clinical Study Group (SBCCSG-03). Breast Cancer Res Treat 107:87–94
Mlineritsch B, Tausch C, Singer C, Luschin-Ebengreuth G, Jakesz R, Ploner F, Stierer M, Melbinger E, Menzel C, Urbania A, Fridrik M, Steger G, Wohlmuth P, Gnant M, Greil R (2008) Exemestane as primary systemic treatment for hormone receptor positive post-menopausal breast cancer patients: a phase II trial of the Austrian Breast and Colorectal Cancer Study Group (ABCSG-17). Breast Cancer Res Treat 112:203–213
Yamashita H, Takahashi S, Ito Y, Yamashita T, Ando Y, Toyama T, Sugiura H, Yoshimoto N, Kobayashi S, Fujii Y, Iwase H (2009) Predictors of response to exemestane as primary endocrine therapy in estrogen receptor-positive breast cancer. Cancer Sci 100:2028–2033
Barnadas A, Gil M, González S, Tusquets I, Munoz M, Arcusa A, Prieto L, Margeli-Vila M, Moreno A (2009) Exemestane as primary treatment of oestrogen receptor-positive breast cancer in postmenopausal women: a phase II trial. Br J Cancer 100:442–449
Miller WR, Dixon JM, Macfarlane L, Cameron D, Anderson TJ (2003) Pathological features of breast cancer response following neoadjuvant treatment with either letrozole or tamoxifen. Eur J Cancer 39:462–468
Dowsett M, Ebbs SR, Dixon JM, Skene A, Griffith C, Boeddinghaus I, Salter J, Detre S, Hills M, Ashley S, Francis S, Walsh G, Smith IE (2005) Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer-a study from the IMPACT trialists. J Clin Oncol 23:2477–2492
Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, A’Hern R, Salter J, Detre S, Hills M, Walsh G (2007) Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167–170
Anderson H, Bulun S, Ian Smith, Dowsett M (2007) Predictors of response to aromatase inhibitors. J Steroid Biochem Mol Biol 106:49–54
Ellis MJ, Coop A, Singh B, Mauriac L, Llombert-Cussac A, Jänicke F, Miller WR, Evans DB, Dugan M, Brady C, Quebe-Fehling E, Borgs M (2001) Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: evidence from a phase III randomized trial. J Cli Oncol 19:3808–3816
Suzuki T, Moriya T, Ariga N, Kaneko C, Kanazawa M, Sasano H (2000) 17beta-hydroxysteroid dehydrogenase type 1 and type 2 in human breast carcinoma: a correlation to clinicopathological parameters. Br J Cancer 82:518–523
Aka JA, Mazumdar M, Lin SX (2009) Reductive 17β-hydroxysteroid dehydrogenases in the sulfatase pathway: Critical in the cell proliferation of breast cancer. Mol Cell Endocrinol 301:183–190
Acknowledgments
We thank the local hospital pathologists who performed tumor sample analysis and other clinicians who were not part of the research team, but took responsibility for patient management. Japanese Foundation for Multidisciplinary Treatment of Cancer was responsible for collating the data in a database. We also thank Kyowa medix co. Ltd Japan for providing a kit of STS antibody.
Disclosure/conflict of interest
Hironobu Sasano and Yasuhiro Miki received the educational research grant from Pfizer Japan Inc., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chanplakorn, N., Chanplakorn, P., Suzuki, T. et al. Increased estrogen sulfatase (STS) and 17β-hydroxysteroid dehydrogenase type 1(17β-HSD1) following neoadjuvant aromatase inhibitor therapy in breast cancer patients. Breast Cancer Res Treat 120, 639–648 (2010). https://doi.org/10.1007/s10549-010-0785-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-0785-3