Abstract
Purpose
It is critical for gastrointestinal cancer researchers and clinicians to have access to comprehensive, sensitive and simple-to-use symptom measures that allow them to understand and quantify the subjective patient experience. Development and validation of such scales requires training in psychometrics and occasionally uses technical jargon that can be difficult to penetrate. This review evaluates existing measures of gastrointestinal cancer symptoms, provides tool descriptions, and uses predefined, objective quality criteria to rate psychometric quality and facilitate tool choices for researchers and clinicians.
Methods
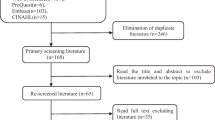
MEDLINE, EMBASE, CINAHL, and PsycINFO databases were systematically reviewed for scales assessing gastrointestinal cancer and gastrointestinal cancer site-specific symptoms. Evaluation criteria were the following: breadth of domain coverage (content validity), high internal consistency (α ≥ .80), sensitivity to change, and extent of validation.
Results
In n = 36 validation studies, 26 gastrointestinal cancer symptom measures were identified. Of these, n = 13 tools met criteria for recommendation, and six in particular showed strong psychometric properties. The Functional Assessment of Cancer Therapy-Colorectal (FACT-C), European Organization for Research and Treatment of Cancer (EORTC) gastric cancer module (QLQ-STO22), FACT-Hepatobiliary (FACT-Hep), and EORTC oesophagus, oesophago-gastric junction and stomach module (QLQ OG-25) were identified as the most comprehensive and best validated scales for each of the major gastrointestinal cancer sites. The FACT-Colorectal Symptom Index (FCSI-9) and the National Comprehensive Cancer Network (NCCN) FACT-Hepatobiliary Symptom Index (FHSI-18) were specifically validated in patients with advanced colorectal and liver cancer and also demonstrated superior psychometric properties.
Conclusions
Several comprehensive, well-validated scales exist to adequately assess gastrointestinal cancer site-specific symptoms. Specifically, gastrointestinal cancer submodules of the FACT quality of life questionnaire represent adequate tool choices in most instances and overall, were better validated than the respective EORTC tools. Further improvement of existing, highly rated measures is recommended.

Similar content being viewed by others
References
Canadian Cancer Society’s Advisory Committee on Cancer Statistics (2013) Canadian Cancer Statistics. Canadian Cancer Society. http://www.cancer.ca/statistics
Cella D, Rosenbloom SK, Beaumont JL et al (2011) Development and validation of eleven symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Cancer Netw 9:269–278
Wang XS, Williams LA, Eng C et al (2010) Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer 116:2053–2063
Fan S, Eiser C, Ho MC (2010) Health-related quality of life in patients with hepatocellular carcinoma: a systematic review. Clin Gastroenterol Hepatol 8:550–564
Parameswaran R, Clifton, JC, Blazeby JM (2010) Quality of life measures in patients with esophageal cancer. In: Handbook of disease burdens and quality of life measures. Springer, New York, pp 2796–807
Mallet S, Deeks JJ, Halligan S et al (2006) Systematic reviews of diagnostic tests in cancer: reviews of methods and reporting. BMJ. doi:10.1136/bmj.38895.467130.55
Mokkink LB, Terwee CB, Patrick DL et al (2010) The COSMIN checklist assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19:539–549
Mokkink LB, Terwee CB, Gibbons E et al (2010) Inter-rater agreement and reliability of the COSMIN (Consensus-based standards for the selection of health status measurement instruments) Checklist. BMC Med Res Methodol 10:82
Streiner DL, Norman GR (2008) Health measurement scales: a practical guide to their development and use. Oxford University Press, New York
Cella D, Paul D, Yount S et al (2003) What are the most important symptom targets when treating advanced cancer? A survey of providers in the national comprehensive cancer network. Cancer Investig 21:526–535
Butt Z, Parikh ND, Beaumont JL et al (2012) Development and validation of a symptom index for advanced hepatobiliary and pancreatic cancers. Cancer. doi:10.1002/cncr.27588
Nakamura M, Kido Y, Yano M, Hosoya Y (2005) Reliability and validity of a new scale to assess postoperative dysfunction after resection of upper gastrointestinal carcinoma. Surg Today 35:535–542
Nakamura M, Kido Y, Egawa T (2008) Development of a 32-item scale to assess postoperative dysfunction after upper gastrointestinal cancer resection. J Clin Nurs 17:1440–1449
Nakamura M, Hosoya Y, Yano M et al (2011) Extent of gastric resection impacts patient quality of life: the Dysfunction After Upper Gastrointestinal Surgery for Cancer (DAUGS32) scoring system. Ann Surg Oncol 18:314–320
Nakamura M, Hosoya Y, Umeshita K et al (2011) Postoperative quality of life: development and validation of the “Dysfunction After Upper Gastrointestinal Surgery” scoring system. J Am Coll Surg 213:508–514
Blazeby JM, Alderson D, Winstone K et al (1996) Development of an EORTC questionnaire module to be used in quality of life assessment for patients with oesophageal cancer. The EORTC Quality of Life Study Group. Eur J Cancer 32A:1912–1917
Blazeby JM, Conroy T, Hammerlid E et al (2003) Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 39:1384–1394
Adelstein BA, Irwig L, Macaskill P et al (2008) A self administered reliable questionnaire to assess lower bowel symptoms. BMC Gastroenterol. doi:10.1186/1471-230X-8-8
Emmertsen KJ, Laurberg S (2012) Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 255:922–928
Gujral S, Conroy T, Fleissner C et al (2007) Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer 43:1564–1573
Whistance RN, Conroy T, Chie W et al (2009) Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 45:3017–3026
Ward WL, Hahn EA, Mo F et al (1999) Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res 8:181–195
Temple LK, Bacik J, Savatta SG et al (2005) The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum 48:1353–1365
Hashimoto H, Shiokawa H, Funahashi K et al (2010) Development and validation of a modified fecal incontinence quality of life scale for Japanese patients after intersphincteric resection for very low rectal cancer. J Gastroenterol 45:928–935
Colwell HH, Mathias SD, Turner MP et al (2010) Psychometric evaluation of the FACT Colorectal Cancer Symptom Index (FCSI-9): reliability, validity, responsiveness, and clinical meaningfulness. Oncologist 15:308–316
Camilleri-Brennan J, Ruta DA, Steele RJ (2002) Patient generated index: new instrument for measuring quality of life in patients with rectal cancer. World J Surg 26:1354–1359
Yang Z, Lu YB, Wan CH et al (2008) Development of the system of quality of life instruments for cancer patients: colorectal cancer (QLICP-CR). Aizheng 27:96–100
Vickery CW, Blazeby JM, Conroy T et al (2001) Development of an EORTC disease-specific quality of life module for use in patients with gastric cancer. Eur J Cancer 37:966–971
Blazeby JM, Conroy T, Bottomley A et al (2004) Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-STO22, to assess quality of life in patients with gastric cancer. Eur J Cancer 40:2260–2268
Lagergren P, Fayers P, Conroy T et al (2007) Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer 43:2066–2073
Clifton JC, Finley RJ, Gelfand G et al (2007) Development and validation of a disease-specific quality of life questionnaire (EQOL) for potentially curable patients with carcinoma of the esophagus. Dis Esophagus 20:191–201
Darling G, Eton DT, Sulman J, Casson AG, Celia D (2006) Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer 107:854–863
Eremenco SL, Cashy J, Webster K et al (2004) FACT-Gastric: a new international measure of QOL in gastric cancer. J Clin Oncol 22:8123
Garland SN, Pelletier G, Lawe A et al (2011) Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy–gastric (FACT-Ga) quality-of-life instrument. Cancer 117:1302–1312
Yeung SM, Shiu AT, Martin CR, Chu KM (2006) Translation and validation of the Chinese version of the Gastrointestinal Quality of Life Index in patients with gastric tumor. J Psychosom Res 61:469–477
Meng Q, Wan CH, Luo JH et al (2008) Development of the system of quality of life instruments for cancer patients: stomach cancer (QLICP-ST). Aizheng 27:1212–1216
Kavadas V, Blazeby JM, Conroy T et al (2003) Development of an EORTC disease-specific quality of life questionnaire for use in patients with liver metastases from colorectal cancer. Eur J Cancer 39:1259–1263
Blazeby JM, Fayers P, Conroy T et al (2009) European Organization for Research Treatment of Cancer (EORTC) Quality of Life Group. Validation of the European Organization for Research and Treatment of Cancer QLQ-LMC21 questionnaire for assessment of patient-reported outcomes during treatment of colorectal liver metastases. Br J Surg 96:291–298
Blazeby JM, Currie E, Zee BC et al (2004) Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 40:2439–2444
Chie WC, Blazeby JM, Hsiao CF et al (2012) International cross-cultural field validation of an European Organization for Research and Treatment of Cancer questionnaire module for patients with primary liver cancer, the European Organization for Research and Treatment of Cancer quality-of-life questionnaire HCC18. Hepatology 55:1122–1129
Heffernan N, Cella D, Webster K et al (2002) Measuring health-related quality of life in patients with hepatobiliary cancers: the Functional Assessment of Cancer Therapy—Hepatobiliary questionnaire. J Clin Oncol 20:2229–2239
Steel JL, Eton DT, Cella D, Olek MC, Carr BI (2006) Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol 17:304–312
Casanovas T, Jane L, Herdman M et al (2010) Assessing outcomes in liver disease patients: reliability and validity of the Spanish version of the Liver Disease Quality of Life Questionnaire (LDQOL 1.0). Value Health 13:455–462
Yount S, Cella D, Webster K et al (2002) Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancer: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manag 24:32–44
Wan C, Fang J, Yang Z et al (2010) Development and validation of a quality of life instrument for patients with liver cancer QOL-LC. Am J Clin Oncol 33:448–455
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Cella DF, Tulsky DS, Gray G et al (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Sprangers MA, te Velde A, Aaronson NK (1999) The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 35:238–247
Uwer L, Rotonda C, Guillemin F et al (2011) Responsiveness of EORTC QLQ-C30, QLQ- CR38 and FACT-C quality of life questionnaires in patients with colorectal cancer. Health Qual Life Out. doi:10.1186/1477-7525-9-70
Bassi C, Johnson C, Fitzsimmons D et al (1999) Quality of life assessment in pancreatic carcinoma: results of an European multicentric study. Chir Ital 51:359–366
Chisholm EM, De Dombal FT, Giles GR (1985) Validation of a self-administered questionnaire to elicit gastrointestinal symptoms. Br Med J 290:1795–1796
Davies A, Larsson G, Ardill J et al (2006) Development of a disease-specific quality of life questionnaire module for patients with gastrointestinal neuroendocrine tumours. Eur J Cancer 42:477–484
Friend E, Yadegarfar G, Byrne C et al (2011) Development of a questionnaire (EORTC module) to measure quality of life in patients with cholangiocarcinoma and gallbladder cancer, the EORTC QLQ-BIL21. Br J Cancer 104:587–592
Nieveen Van Dijkum EJ, Terwee CB et al (2000) Validation of the gastrointestinal quality of life index for patients with potentially operable periampullary carcinoma. Br J Surg 87:110–115
Disclosures and acknowledgments
No funding was received for this manuscript. None of the authors are in any financial or personal conflict of interest regarding this work. We are very grateful for the feedback of Dr. Jessica McAlpine on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A
(PDF 44.5 kb)
Rights and permissions
About this article
Cite this article
Pullmer, R., Linden, W., Rnic, K. et al. Measuring symptoms in gastrointestinal cancer: a systematic review of assessment instruments. Support Care Cancer 22, 2941–2955 (2014). https://doi.org/10.1007/s00520-014-2250-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-014-2250-z