Abstract
Background
Chemotherapy-induced nausea and vomiting (CINV) are some of the most problematic symptoms for cancer patients. Triplet therapy consisting of a 5HT3 receptor antagonist, aprepitant, and dexamethasone is a guideline-recommended antiemetic prophylaxis for highly emetogenic chemotherapy (HEC). The efficacy and safety of triplet therapy using a 0.75-mg dose of palonosetron have not yet been investigated. We performed a prospective phase II study using triplet antiemetic therapy with 0.75 mg of palonosetron.
Methods
Chemotherapy-naïve lung cancer patients scheduled to receive HEC were enrolled. The eligible patients were pretreated with antiemetic therapy consisting of the intravenous administration of 0.75 mg of palonosetron, and 9.9 mg of dexamethasone and the oral administration of 125 mg of aprepitant on day 1, followed by the oral administration of 80 mg of aprepitant on days 2–3 and the oral administration of 8 mg of dexamethasone on days 2–4. The primary endpoint was the complete response rate (the CR rate; no vomiting and no rescue medication) during the overall phase (0–120 h).
Results
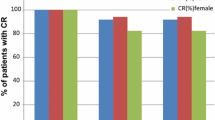
The efficacy analysis was performed in 63 patients. The CR rates during the overall, acute and delayed phases were 81.0, 96.8, and 81.0 %, respectively. The no nausea and no significant nausea rate during the overall phase were 54.0 and 66.7 %, respectively. The most common adverse event was grade 1 or 2 constipation.
Conclusions
Triplet antiemetic therapy using a 0.75-mg dose of palonosetron shows a promising antiemetic effect in preventing CINV in lung cancer patients receiving HEC.
Similar content being viewed by others
References
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH (2011) Antiemetics: American Society of Clinical Oncology Clinical Practice guideline update. J Clin Oncol 29:4189–4198
Darmani NA, Chebolu S, Amos B, Alkam T (2011) Synergistic antiemetic interactions between serotonergic 5-HT3 and tachykininergic NK1-receptor antagonists in the least shrew (Cryptotis parva). Pharmacol Biochem Behav 99:573–579
Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suarez C et al (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20(12):3141–3148. doi:10.1007/s00520-012-1448-1, Epub 2012 Mar 31
Herrington JD, Jaskiewicz AD, Song J (2008) Randomized, placebo-controlled, pilot study evaluating aprepitant single dose plus palonosetron and dexamethasone for the prevention of acute and delayed chemotherapy-induced nausea and vomiting. Cancer 112:2080–2087
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol 21:4112–4119
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol 15:103–109
Likun Z, Xiang J, Yi B, Xin D, Tao ZL (2011) A systematic review and meta-analysis of intravenous palonosetron in the prevention of chemotherapy-induced nausea and vomiting in adults. Oncologist 16:207–216
Longo F, Mansueto G, Lapadula V, De Sanctis R, Quadrini S, Grande R, Gori B, Altavilla A, D’Antoni I, Del Signore E, Stumbo L, De Luca C, Cimadon B, Cortesi E, Gamucci T, Di Seri M (2011) Palonosetron plus 3-day aprepitant and dexamethasone to prevent nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 19:1159–1164
Lorusso V, Spedicato A, Petrucelli L, Saracino V, Giampaglia M, Perrone T (2009) Single dose of palonosetron plus dexamethasone to control nausea, vomiting and to warrant an adequate food intake in patients treated with highly emetogenic chemotherapy (HEC). Prelim results Support Care Cancer 17:1469–1473
Maemondo M, Masuda N, Sekine I, Kubota K, Segawa Y, Shibuya M, Imamura F, Katakami N, Hida T, Takeo S (2009) A phase II study of palonosetron combined with dexamethasone to prevent nausea and vomiting induced by highly emetogenic chemotherapy. Ann Oncol 20:1860–1866
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in. Lat Am Cancer 97:3090–3098
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(5):232–243
Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, Cantoreggi S, Sebastiani S, Pietra C, Slusher BS (2010) The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335:362–368
Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, Sebastiani S, Cantoreggi S, Slusher BS (2010) Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626:193–199
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Schmoll HJ, Aapro MS, Poli-Bigelli S, Kim HK, Park K, Jordan K, von Pawel J, Giezek H, Ahmed T, Chan CY (2006) Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17:1000–1006
Segawa Y, Aogi K, Inoue K, Sano M, Sekine I, Tokuda Y, Isobe H, Ogura T, Tsuboi M, Atagi S (2009) A phase II dose-ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide-based chemotherapy. Ann Oncol 20:1874–1880
Stoltz R, Cyong JC, Shah A, Parisi S (2004) Pharmacokinetic and safety evaluation of palonosetron, a 5-hydroxytryptamine-3 receptor antagonist, in US and Japanese healthy subjects. J Clin Pharmacol 44:520–531
Acknowledgments
This study was conducted by the Niigata Lung Cancer Treatment Group. The authors are thankful to the participating institutions and the following collaborating investigators: Koh Sato, Satoshi Hokari, Nao Koshio, Jun Koshio, Kosuke Ichikawa, and Toshiki Furukawa (Niigata University Medical and Dental Hospital); Masachika Hayashi and Hideki Kuriyama (Nagaoka Red Cross Hospital); Junichi Tanaka and Akira Hirata (Niigata Prefectural Shibata Hospital); Katsunori Kawamkami, Takahiro Miyabayashi, and Takashi Ishida (Niigata Prefectural Central Hospital); Junko Baba, Yoshiki Hayashi, Toru Hiura, and Tetsuya Abe (Niigata Cancer Center Hospital); Takeshi Ota and Akira Ishida (Nagaoka Chuo General Hospital); Naoya Matsumoto (Nishi-Niigata Chuo National Hospital); Syotetsu Kawabe and Masaki Terada (Saiseikai Niigata Daini Hospital); Yuka Kimura, Takafumi Tezuka, Kazuhiko Ito, and Hiroki Tsukada (Niigata City General Hospital); Yuichi Shimaoka (Niigata Prefectural Koide Hospital); and Koki Matsuyama (Niigata Prefectural Yoshida Hospital). We would like to thank Kumiko Shirai from the Bioscience Medical Research Center at Niigata University Medical and Dental Hospital for her support in data collection and management.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, S., Watanabe, S., Sato, K. et al. The efficacy of triplet antiemetic therapy with 0.75 mg of palonosetron for chemotherapy-induced nausea and vomiting in lung cancer patients receiving highly emetogenic chemotherapy. Support Care Cancer 21, 2575–2581 (2013). https://doi.org/10.1007/s00520-013-1835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1835-2