Abstract
Purpose
Olanzapine has been shown to be a safe and effective agent for the prevention of chemotherapy-induced nausea and vomiting (CINV). Olanzapine may also be an effective rescue medication for patients who develop breakthrough CINV despite having received guideline-directed CINV prophylaxis.
Methods
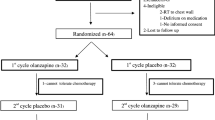
A double-blind, randomized phase III trial was performed for the treatment of breakthrough CINV in chemotherapy-naive patients receiving highly emetogenic chemotherapy (cisplatin, ≥ 70 mg/m2 or doxorubicin, ≥ 50 mg/m2 and cyclophosphamide, ≥ 600 mg/m2), comparing olanzapine to metoclopramide. Patients who developed breakthrough emesis or nausea despite prophylactic dexamethasone (12 mg IV), palonosetron (0.25 mg IV), and fosaprepitant (150 mg IV) pre-chemotherapy and dexamethasone (8 mg p.o. daily, days 2–4) post-chemotherapy were randomized to receive olanzapine, 10 mg orally daily for 3 days or metoclopramide, 10 mg orally TID for 3 days. Patients were monitored for emesis and nausea for 72 h after taking olanzapine or metoclopramide. Two hundred seventy-six patients (median age 62 years, range 38–79; 43 % women; Eastern Cooperative Oncology Group (ECOG) PS 0,1) consented to the protocol. One hundred twelve patients developed breakthrough CINV and 108 were evaluable.
Results
During the 72-h observation period, 39 out of 56 (70 %) patients receiving olanzapine had no emesis compared to 16 out of 52 (31 %) patients with no emesis for patients receiving metoclopramide (p < 0.01). Patients without nausea (0, scale 0–10, M.D. Anderson Symptom Inventory) during the 72-h observation period were those who took olanzapine, 68 % (38 of 56), and metoclopramide, 23 % (12 of 52) (p < 0.01). There were no grade 3 or 4 toxicities.
Conclusions
Olanzapine was significantly better than metoclopramide in the control of breakthrough emesis and nausea in patients receiving highly emetogenic chemotherapy.


Similar content being viewed by others
References
Bloechl-Daum B, Deuson RR, Panagiotis M et al (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Navari RM (2009) Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs 69:515–533
Navari RM (2010) Palonosetron for the prevention of chemotherapy-induced nausea and vomiting in patients with cancer. Future Oncol 6:1073–1084
Curran MP, Robinson DM (2009) Aprepitant: a review of its use in the prevention of nausea and vomiting. Drugs 69:1853–1858
Sankhala KK, Pandya DM, Sarantopoulos J et al (2009) Prevention of chemotherapy induced nausea and vomiting: a focus on aprepitant. Expert Opin Drug Metab Toxicol 12:1607–1614
Navari RM, Einhorn LH, Loehrer PJ et al (2007) A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting. Support Care Canc 15:1285–1291
Tan L, Liu J, Liu X et al (2009) Clinical research of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 28:1–7
Navari RM, Gray SE, Kerr AC (2011) Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol 9:188–195
Cohen L, deMoor CA, Eisenberg P et al (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Canc 15:497–503
Tina Shih YC, Xu Y, Elting LS (2007) Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer 110:678–685
Grunberg SM, Deuson RR, Mavros P et al (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100:2261–2268
Hickok JT, Roscoe JA, Morrow GR et al (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics. Cancer 97:2880–2886
Roila F, Herrstedt J, Aapro M et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):232–243
Basch E, Prestrud AP, Hesketh PJ et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29:4189–4198
NCCN (2012) NCCN clinical practice guidelines in oncology. Antiemesis version 1. http://www.nccn.org. Accessed 15 Nov 2012
Kris MC (2003) Why do we need another antiemetic? Just ask. J Clin Oncol 21:4077–4080
National Cancer Institute (2012) www.cancer.gov/cancertopics/pdq/supportivecare/nausea/healthprofessional. Accessed 15 Nov 2012
Wales J, Sanger L (2001) www.wikipedia.org/wiki/Metoclopramide. Accessed 1 Nov 2012
Bymaster FP, Calligaro D, Falcone J et al (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Bymaster FP, Falcone JF, Bauzon D et al (2001) Potent antagonism of 5HT3 and 5HT6 receptors by olanzapine. Eur J Pharmacol 430:341–349
Allison DB, Casey DE (2001) Antipsychotic-associated weight gain: a review of the literature. J Clin Psychiatr 62:22–31
Hale AS (1997) Olanzapine. Br J Hosp Med 58:443–445
Goldstein LE, Sporn J, Brown S et al (1999) New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 40:438–443
Cleeland CS, Mendoza TR, Wang XS et al (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89:1634–1646
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study group. J Clin Oncol 21:4112–4119
Longo F, Mansuetto G, Lapadula V et al (2012) Combination of aprepitant, palonosetron and dexamethasone as antiemetic prophylaxis in lung cancer patients receiving multiple cycles of cisplatin-based chemotherapy. Int J Clin Pract 66:753–757
Hesketh PJ, Navari RM, Grote T et al (1996) Double blind randomized comparison of the antiemetic efficacy of intravenous dolasetron and intravenous ondansetron in the prevention of acute cisplatin-induced emesis in patients with cancer. J Clin Oncol 14:2242–2249
Navari RM, Gandara D, Hesketh PJ et al (1995) Comparative clinical trial of granisetron and ondansetron in the prophylaxis of cisplatin-induced emesis. J Clin Oncol 13:1242–1248
Hulstaert F, VanBelle S, Bleiberg H et al (1994) Optimal combination therapy with tropisetron in 445 patients with incomplete control of chemotherapy-induced nausea and vomiting. J Clin Oncol 12:2439–2446
The Italian Group for Antiemetic Research (2000) Dexamethasone alone or in combination with ondanseteron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 342:1554–1559
Jones JM, Qin R, Bardia A et al (2011) Antiemetics for chemotherapy-induced nausea and vomiting occurring despite prophylactic antiemetic therapy. J Palliat Med 14:810–814
Bleicher J, Bhaskara A, Huyck T et al (2008) Lorazepam, diphenhydramine, and haloperidol transdermal gel for rescue from chemotherapy-induced nausea/vomiting: results from two pilot trials. J Support Oncol 6:27–32
Passik SD, Lundberg J, Kirsh K et al (2002) A pilot exploration of the antiemetic activity of olanzapine (Zyprexa) for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manag 23:526–532
Pirl WF, Roth AJ (2000) Remission of chemotherapy-induced emesis with concurrent olanzapine treatment: a case report. Psychooncology 9:84–87
Jackson WC, Tavernier L (2003) Olanzapine for intractable nausea in palliative care patients. J Palliat Med 6:251–255
Srivastava M, Brito-Dellan N, Davis MP et al (2003) Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manag 25:578–582
Rudd JA, Ngan MP, Wai MK et al (2006) Antiemetic activity of ghrelin in ferrets exposed to the cytotoxic anti-cancer agent cisplatin. Neurosci Lett 393:79–83
Yakabi K, Sadakane C, Noguchi M et al (2010) Reduced ghrelin secretion in the hypothalamus of rats due to cisplatin-induced anorexia. Endocrinology 151:3773–3782
Ithimakin S, Runglodvatana K, Nimmannit A et al (2012) Randomized, double-blinded, placebo-controlled trial of ondansetron plus dexamethasone with or without metoclopramide as antiemetic prophylaxis in patients receiving high-dose cisplatin in medical practice. Support Care Canc 20:849–855
Acknowledgments
This study is supported by the Reich Family Endowment for the Care of the Whole Patient.
Conflict of interest
None of the authors have a financial relationship with the sponsoring organization, and none of the authors have any conflict of interest. The authors have full control of the primary data and allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navari, R.M., Nagy, C.K. & Gray, S.E. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 21, 1655–1663 (2013). https://doi.org/10.1007/s00520-012-1710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-012-1710-6