Abstract
Purpose
Prevention of chemotherapy-induced nausea and vomiting (CINV) is of great importance for the completion of multiple cycles of cancer chemotherapy. Palonosetron is a second-generation 5-HT3 receptor antagonist with proven efficacy for both acute and delayed CINV. This study was designed to assess the safety and efficacy of 0.75 mg palonosetron in repeated cycles of highly emetogenic chemotherapy or anthracycline–cyclophosphamide combination (AC/EC).
Methods
We gave 0.75 mg palonosetron to 538 patients 30 min prior to ≥50 mg/m2 cisplatin or AC/EC on day 1. Prophylactic dexamethasone was administered on days 1–3. The primary endpoint was the incidence rate of adverse events (AEs). The secondary endpoint was complete response rate (CR, defined as no emesis and no rescue medication) throughout the study period.
Results
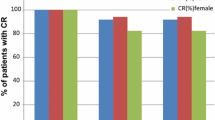
Treatment-related AEs were seen in 44% (237 of 538 patients). Serious AEs were seen in 4% (23 of 538 patients), all considered unrelated or unlikely to be related to palonosetron. Only one patient discontinued the study due to a treatment-related AE. No trend toward worsening of AEs was observed in subsequent cycles of chemotherapy. Complete response rates were maintained throughout repeated cycles.
Conclusion
The extraordinary safety profile and maintenance of efficacy of 0.75 mg palonosetron combined with dexamethasone were demonstrated throughout repeated chemotherapy cycles.

Similar content being viewed by others
References
de Boer-Dennert M, de Wit R, Schmitz PI, Djontono J, v Beurden V, Stoter G, Verweij J (1997) Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer 76:1055–1061
Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, Sawyer WT (1999) Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 7:59–65
Sun CC, Bodurka DC, Weaver CB, Rasu R, Wolf JK, Bevers MW, Smith JA, Wharton JT, Rubenstein EB (2005) Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer 13:219–227
Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:2473–2482
Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Maemondo M, Masuda N, Sekine I, Kubota K, Segawa Y, Shibuya M, Imamura F, Katakami N, Hida T, Takeo S (2009) A phase II study of palonosetron combined with dexamethasone to prevent nausea and vomiting induced by highly emetogenic chemotherapy. Ann Oncol 20:1860–1866
Segawa Y, Aogi K, Inoue K, Sano M, Sekine I, Tokuda Y, Isobe H, Ogura T, Tsuboi M, Atagi S (2009) A phase II dose-ranging study of palonosetron in Japanese patients receiving moderately emetogenic chemotherapy, including anthracycline and cyclophosphamide-based chemotherapy. Ann Oncol 20:1874–1880
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10:115–124
Cartmell A, Ferguson S, Yanagihara R, Moiseyenko V, RvM K, Tripp F, Macciocchi A (2003) Protection against chemotherapy-induced nausea and vomiting (CINV) is maintained over multiple cycles of moderately or highly emetogenic chemotherapy by palonosetron (PALO), a potent 5-HT3 receptor antagonist (RA). Proc Amer Soc Clin Oncol 22:776
Ettinger DS, Bierman PJ, Bradbury B, Comish CC, Ellis G, Ignoffo RJ, Kirkegaard S, Kloth DD, Kris MG, Lim D, Markiewicz MA, McNulty R, Nabati L, Todaro B, Urba S, Yowell S (2007) Antiemesis. J Natl Compr Canc Netw 5:12–33
Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243
Ohmatsu H, Eguchi K, Shinkai T, Tamura T, Ohe Y, Nisio M, Kunikane H, Arioka H, Karato A, Nakashima H et al (1994) A randomized cross-over study of high-dose metoclopramide plus dexamethasone versus granisetron plus dexamethasone in patients receiving chemotherapy with high-dose cisplatin. Jpn J Cancer Res 85:1151–1158
Sekine I, Nishiwaki Y, Kakinuma R, Kubota K, Hojo F, Matsumoto T, Ohmatsu H, Yokozaki M, Kodama T (1996) A randomized cross-over trial of granisetron and dexamethasone versus granisetron alone: the role of dexamethasone on day 1 in the control of cisplatin-induced delayed emesis. Jpn J Clin Oncol 26:164–168
Kuryshev YA, Brown AM, Wang L, Benedict CR, Rampe D (2000) Interactions of the 5-hydroxytryptamine 3 antagonist class of antiemetic drugs with human cardiac ion channels. J Pharmacol Exp Ther 295:614–620
Morganroth J, Parisi S, Moresino C, Thorn M, Cullen MT (2007) High dose palonosetron does not alter ECG parameters including QTc interval in healthy subjects: results of a dose-response, double blind, randomized, parallel E14 study of palonosetron vs. moxifloxacin or placebo. Eur J Cancer Suppl 5:158–159
de Wit R, van den Berg H, Burghouts J, Nortier J, Slee P, Rodenburg C, Keizer J, Fonteyn M, Verweij J, Wils J (1998) Initial high anti-emetic efficacy of granisetron with dexamethasone is not maintained over repeated cycles. Br J Cancer 77:1487–1491
Acknowledgments
The authors thank Mamoru Tsukuda (Yokohama City University School of Medicine) for his advice on the creation of the protocol, assessment, and handling of safety information and all other issues relating to the study; Chikuma Hamada (Tokyo University of Science) for his advice and guidance from the statistical standpoint; and Yutaka Ariyoshi (Aichi Cancer Center, Aichi Hospital), Tomohide Tamura (National Cancer Center), and Toshiaki Saeki (Saitama Medical University) for coordination of this multicenter study between investigational sites, including the handling of questions concerning the interpretation of the protocol. The authors are also grateful to Prof. J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University, a remunerated consultant of Taiho pharmaceutical Co., Ltd., for his review of this manuscript.
Funding
Funding was provided by Taiho Pharmaceutical Co., Ltd.
Disclosures
From Taiho Pharmaceutical, K.A. has received lecture fees, H.Y. has received grants, and N.K. has received research funds. The preliminary results of this study were presented in part at the European Society of Medical Oncology, Stockholm, Sweden, September 2008.
Principal investigators at trial sites
The following were principal investigators at trial sites: Akira Inoue (Tohoku University Hospital, Sendai, Japan); Makoto Maemondo (Miyagi Cancer Centre, Natori, Japan); Akira Yokoyama (Niigata Cancer Centre Hospital, Niigata, Japan); Hirohisa Yoshizawa (Niigata University Medical and Dental Hospital, Niigata, Japan); Koichi Minato and Yasuhiro Yanagita (Gunma Cancer Centre, Ota, Japan); Kiyoshi Mori (Tochigi Cancer Centre, Utsunomiya, Japan); Shoichi Mitsuhashi (Ibaraki Prefectural Central Hospital and Cancer Centre, Kasama, Japan); Hiroshi Sakai and Kenichi Inoue (Saitama Cancer Centre, Inamachi, Japan); Yasutsuna Sasaki (Saitama Medical University Hospital, Moroyama, Japan); Yuichi Takiguchi (Chiba University Hospital, Chiba, Japan); Kozo Yoshimori (Fukujuji Hospital, Kiyose, Japan); Tomoyuki Goya (Kyorin University Hospital, Mitaka, Japan); Masahiko Shibuya, Masakazu Toi, and Shigehira Saji (Tokyo Metropolitan Cancer and Infectious Diseases Centre, Komagome Hospital, Tokyo, Japan); Yuichiro Takeda and Hidemitsu Yasuda (International Medical Centre of Japan, Tokyo, Japan); Masahiro Tsuboi (Tokyo Medical University Hospital, Tokyo, Japan); Ikuo Sekine and Yasuhiro Fujiwara (National Cancer Centre Hospital, Tokyo, Japan); Takashi Ogura (Kanagawa Cardiovascular and Respiratory Centre, Yokohama, Japan); Noriyuki Masuda and Masaru Kuranami (Kitasato University Hospital, Sagamihara, Japan); Yutaka Tokuda (Tokai University Hospital, Isehara, Japan); Hiroaki Okamoto and Akira Ishiyama (Yokohama Municipal Citizen's Hospital, Yokohama, Japan); Hitoshi Arioka (Yokohama Rosai Hospital, Yokohama, Japan); Kazuo Kasahara (Kanazawa University Hospital, Kanazawa, Japan); Koichi Nishi (Ishikawa Prefectural Central Hospital, Kanazawa, Japan); Masahiro Endo (Shizuoka Cancer Centre, Nagaizumi, Japan); Kazuhiko Nakagami (Shizuoka General Hospital, Shizuoka, Japan); Toyoaki Hida (Aichi Cancer Centre Central Hospital, Nagoya, Japan); Yoshinori Hasegawa (Nagoya University Hospital, Nagoya, Japan); Chiyoe Kitagawa (National Hospital Organization Nagoya Medical Centre, Nagoya, Japan); Hiroshi Saito and Mitsuhiro Mizutani (Aichi Cancer Centre Aichi Hospital, Okazaki, Japan); Jo Shindo (Ogaki Municipal Hospital, Ogaki, Japan); Hironori Kato and Hiroyasu Yamashiro (Kyoto University Hospital, Kyoto, Japan); Fumio Imamura (Osaka Prefectural Hospital Organization Osaka Medical Centre for Cancer and Cardiovascular Diseases, Osaka, Japan); Shinji Atagi (National Hospital Organization Kinki-chuo Chest Medical Centre, Sakai, Japan); Kazuhiko Nakagawa (Kinki University Hospital, Osakasayama, Japan); Norikazu Masuda (National Hospital Organization Osaka National Hospital, Osaka, Japan); Koji Takeda (Osaka City General Hospital, Osaka, Japan); Takashi Nishimura (Kobe City Medical Centre General Hospital, Kobe, Japan); Nobuyuki Katakami (Institute of Biomedical Research and Innovation Hospital, Kobe, Japan); Shunichi Negoro, Morihito Okada, and Koichiro Iwanaga (Hyogo Cancer Centre, Akashi, Japan); Masahiro Tabata (Okayama University Hospital, Okayama, Japan); Yoshihiko Segawa and Kenjiro Aogi (National Hospital Organization Shikoku Cancer Centre, Matsuyama, Japan); Yukito Ichinose and Shinji Ohno (National Hospital Organization Kyushu Cancer Centre, Fukuoka, Japan); Sadanori Takeo and Masafumi Yamaguchi (National Hospital Organization Kyushu Medical Centre, Fukuoka, Japan); Isao Goto (Osaka Medical College Hospital, Takatsuki, Japan); and Shigeru Murakami (Hiroshima University Hospital, Hiroshima, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aogi, K., Sakai, H., Yoshizawa, H. et al. A phase III open-label study to assess safety and efficacy of palonosetron for preventing chemotherapy-induced nausea and vomiting (CINV) in repeated cycles of emetogenic chemotherapy. Support Care Cancer 20, 1507–1514 (2012). https://doi.org/10.1007/s00520-011-1239-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-011-1239-0