Abstract
Background
Pain relief is an important treatment goal for breast cancer patients with metastatic bone disease and treatment should be associated with a low rate of side effects. This interim analysis of a prospective non-interventional study documents the efficacy and safety of the amino-bisphosphonate ibandronate in the treatment of metastatic bone disease under real-life conditions.
Patients and methods
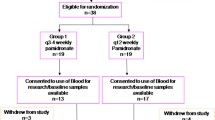
For up to 24 weeks 913 breast cancer patients received IV infusions of 6 mg ibandronate every 3–4 weeks or 50 mg of oral ibandronate once daily. Efficacy variables included pain severity, analgesic use, and skeletal-related events; the major safety parameter was renal function, assessed by serum creatinine levels. Subgroup analyses were performed according to pretreatment with bisphosphonates (none, ibandronate, or other bisphosphonates).
Results
At baseline, patients with ibandronate pretreatment tended to have lower mean pain scores and lower serum creatinine levels than those pre-treated with other bisphosphonates. Over the observation period, analgesic use did not increase. Among the 712 patients reporting pain at baseline, 70% achieved an improvement in pain severity during treatment with ibandronate, and there was no evidence to suggest relevant differences in mean pain reductions with IV or oral administration of ibandronate or according to prior bisphosphonate treatment. Skeletal-related events were rare (7%). Changes in serum creatinine levels during ibandronate treatment were small and both formulations of ibandronate were rated as well tolerated by physicians and patients.
Conclusions
Data from this non-interventional study confirm the analgesic efficacy and safety profile of IV and oral ibandronate under real-life conditions.

Similar content being viewed by others
Notes
Funding for this study was provided by Roche Pharma AG, Grenzach, Germany.
References
World Health Organization (1996) WHO Guidelines: cancer pain relief. World Health Organization, Geneva
Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thurlimann B (2008) Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 19:420–432
Body JJ, Diel IJ, Bell R, Pecherstorfer M, Lichinitser MR, Lazarev AF, Tripathy D, Bergstrom B (2004) Oral ibandronate improves bone pain and preserves quality of life in patients with skeletal metastases due to breast cancer. Pain 111:306–312
Body JJ, Diel IJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, Budde M, Bergstrom B (2003) Intravenous ibandronate reduces the incidence of skeletal complications in patients with breast cancer and bone metastases. Ann Oncol 14:1399–1405
Body JJ, Diel IJ, Lichinitzer M, Lazarev A, Pecherstorfer M, Bell R, Tripathy D, Bergstrom B (2004) Oral ibandronate reduces the risk of skeletal complications in breast cancer patients with metastatic bone disease: results from two randomised, placebo-controlled phase III studies. Br J Cancer 90:1133–1137
Chang JT, Green L, Beitz J (2003) Renal failure with the use of zoledronic acid. N Engl J Med 349:1676–1679 discussion 1676–1679
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Conte PF, Latreille J, Mauriac L, Calabresi F, Santos R, Campos D, Bonneterre J, Francini G, Ford JM (1996) Delay in progression of bone metastases in breast cancer patients treated with intravenous pamidronate: results from a multinational randomized controlled trial. The Aredia Multinational Cooperative Group. J Clin Oncol 14:2552–2559
Diel IJ (2007) Effectiveness of bisphosphonates on bone pain and quality of life in breast cancer patients with metastatic bone disease: a review. Support Care Cancer 15:1243–1249
Diel IJ, Body JJ, Lichinitser MR, Kreuser ED, Dornoff W, Gorbunova VA, Budde M, Bergstrom B (2004) Improved quality of life after long-term treatment with the bisphosphonate ibandronate in patients with metastatic bone disease due to breast cancer. Eur J Cancer 40:1704–1712
Ernst DS, MacDonald RN, Paterson AH, Jensen J, Brasher P, Bruera E (1992) A double-blind, crossover trial of intravenous clodronate in metastatic bone pain. J Pain Symptom Manage 7:4–11
Gainford MC, Dranitsaris G, Clemons M (2005) Recent developments in bisphosphonates for patients with metastatic breast cancer. Brit Med J 330:769–773
Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD (1996) Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med 335:1785–1791
Huskisson EC (1974) Measurement of pain. Lancet 2:1127–1131
Johnson KB, Gable P, Kaime EM, Luiken G, Castillos T, Hu J (2003) Significant deterioration in renal function with the new bisphosphonate, zoledronic acid. Proc Am Soc Clin Oncol 22: abstr 2968
O'Rourke N, McCloskey E, Houghton F, Huss H, Kanis JA (1995) Double-blind, placebo-controlled, dose-response trial of oral clodronate in patients with bone metastases. J Clin Oncol 13:929–934
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Pal SK, Gupta R, Bernstein L, Mortimer J (2008) Lack of survival benefit in metastatic breast cancer with newer chemotherapy agents: The City of Hope cancer experience. ASCO 2008 Breast Cancer Symposium; Washington, DC. Abstract #95
Pavlakis N, Schmidt R, Stockler M (2005) Bisphosphonates for breast cancer. Cochrane Database Syst Rev: CD003474
Pecherstorfer M, Rivkin S, Body JJ, Diel I, Bergstrom B (2006) Long-term safety of intravenous ibandronic acid for up to 4 years in metastatic breast cancer: an open-label trial. Clin Drug Investig 26:315–322
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein M, Coleman RE, Reitsma DJ, Seaman JJ, Chen BL, Ambros Y (2001) Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, comparative trial. Cancer J 7:377–387
Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma DJ, Chen BL, Seaman JJ (2003) Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 98:1735–1744
Acknowledgements
We are indebted to the 157 investigators who documented the patients in this trial and thank Gisela Beyendorff-Hajda of Physicians World GmbH for medical writing assistance. The study was supported by Roche Pharma AG, Grenzach-Wyhlen, Germany, who also provided funding for medical writing support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diel, I.J., Kurth, A.H.A., Sittig, HB. et al. Bone pain reduction in patients with metastatic breast cancer treated with ibandronate–results from a post-marketing surveillance study. Support Care Cancer 18, 1305–1312 (2010). https://doi.org/10.1007/s00520-009-0749-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-009-0749-5