Abstract
Objective
The purpose of this study is to determine the control of acute and delayed chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately emetogenic chemotherapy (MEC) and highly emetogenic chemotherapy (HEC) with the combined use of palonosetron and olanzapine, and dexamethasone with the dexamethasone given on day 1 only.
Materials and methods
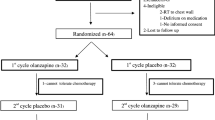
Forty chemotherapy-naive patients received on the day of chemotherapy, day 1, an anti-emetic regimen consisting of dexamethasone, palonosetron, and olanzapine. Patients continued olanzapine for days 2–4 after chemotherapy administration. Patients recorded daily episodes of emesis, daily symptoms utilizing the M.D. Anderson Symptom Inventory, and the utilization of rescue therapy.
Results
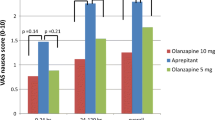
For the first cycle of chemotherapy, the complete response (no emesis, no rescue) for the acute period (24 h post-chemotherapy) was 100%, the delayed period (days 2–5 post-chemotherapy) 75%, and the overall period (0 120 h post-chemotherapy) 75% in 8 patients receiving HEC and was 97, 75, and 72% in 32 patients receiving MEC. Patients with no nausea for the acute period was 100%, the delayed period 50%, and the overall period 50% in 8 patients receiving HEC and was 100, 78, and 78% in 32 patients receiving MEC.
Discussion
The complete response and control of nausea in subsequent cycles of chemotherapy were not significantly different from cycle one.
Conclusion
Olanzapine combined with a single dose of dexamethasone and a single dose of palonosetron was very effective in controlling acute and delayed CINV in patients receiving both HEC and MEC.


Similar content being viewed by others
References
Aapro MS, Grunberg SM, Manikhas GM et al (2006) A phase III, double blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17:1441–1449
Allison DB, Casey DE (2001) Antipsychotic-associated weight gain: a review of the literature. J Clin Psychiatry 62:22–31
Bloechl-Daum B, Deuson RR, Panagiotis M et al (2006) Delayed nausea and vomiting continue to reduce patients’ quality of life alter highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol 24:4472–4478
Bymaster FP, Calligaro D, Falcone J et al (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Bymaster FP, Falcone JF, Bauzon D et al (2001) Potent antagonism of 5HT3 and 5HT6 receptors by olanzapine. Eur J Pharmacol 430:341–349
Cleeland CS, Mendoza TR, Wang XS et al (2000) Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer 89:1634–1646
Eisenberg P, Figueroa-Vadillo J, Zamora R et al (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5HT3 receptor antagonist: results of a phase III, single dose trial versus dolasetron. Cancer 98:2473–2482
Eisenberg P, MacKintosh FR, Ritch P et al (2004) Efficacy, safety, and pharmacokinetics of palonosetron in patients receiving highly emetogenic, cisplatin-based chemotherapy: a dose-ranging, clinical study. Ann Oncol 15:330–337
Fisch MJ, Kim HF (2004) Use of atypical antipsychotic agents for symptom control in patients with advanced cancer. J Support Oncol 2:447–452
Goldstein LE, Sporn J, Brown S et al (1999) New-onset diabetes mellitus and diabetic ketoacidosis associated with olanzapine treatment. Psychosomatics 40:438–443
Gralla R, Lichinitser M, Van der Vegt S et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase II trial comparing single does of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Grunberg SM, Deuson R, Mavros P et al (2004) Incidence of chemotherapy-induced nausea and emesis after modern anti-emetics: perception versus reality. Cancer 100:2261–2268
Hale AS (1997) Olanzapine. Br J Hosp Med 58:443–445
Hesketh PJ, Grunberg SM, Gralla RJ et al (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind placebo-controlled trial in patients receiving high-dose cisplatin-the Aprepitant Protocol 052 Study group. J Clin Oncol 21:4112–4119
Mantovani G, Maccio A, Aslexandro B et al (1996) Comparison of granisetron versus ondansetron versus tropisetron in the prophylaxis of acute nausea and vomiting induced by cisplatin for the treatment of head and neck cancer; a randomized controlled trial. Cancer 77:941–948
Navari RM (2003) Pathogenesis-based treatment of chemotherapy-induced nausea and vomiting: two new agents. J Support Oncol 1:89–103
Navari RM (2004) Inhibiting substance P pathway for prevention of chemotherapy-induced emesis: preclinical data, clinical trials of neurokinin-1 receptor antagonists. Support Cancer Ther 1:89–96
Navari RM (2006) Palonosetron: a second generation 5-hydroxytryptamine receptor antagonist. Future Oncol 2:591–602
Navari RM, Einhorn LH, Loehrer PJ et al (2005) A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting. Support Care Cancer 13:529–534
Navari RM, Reinhardt RR, Gralla RJ et al (1999) Reduction of cisplatin-induced emesis by a selective neurokin-1 receptor antagonist. N Engl J Med 340:190–195
Passik SD, Kirsh KL, Theobald DE et al (2003) A retrospective chart review of the use of olanzapine for the prevention of delayed emesis in cancer patients. J Pain Symptom Manage 25:485–489
Passik SD, Navari RM, Loehrer PJ et al (2004) A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients receiving chemotherapy. Cancer Invest 22:383–388
Pirl WF, Roth AJ (2000) Remission of chemotherapy-induced emesis with concurrent olanzapine treatment: a case report. Psych-Oncology 9:84–87
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD et al (2003) Addition of the neurokinin-1 receptor antagonist Aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Cancer 97:3090–3098
Red Book Annual Drug Topic (2006) Medical Economics Company, Montvale, NJ, pp 239–243
Schmoll HJ, Aapro MS, Poli-Bigelli S et al (2006) Comparison of an aprepitant regime with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol 17:1000–1006
The Italian Group for Antiemetic Research (2000) Dexamethasone alone or in combination with ondansetron for the prevention of delayed nausea and vomiting induced by chemotherapy. N Engl J Med 342:1554–1559
Vardy J, Chiew KS, Gallica J et al (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94:1011–1015
Warr DG, Hesketh PJ, Gralla RJ et al (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol 23:2822–2830
Acknowledgment
This study was supported by the Walther Cancer Institute, MGI Pharma, and Eli Lilly.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Navari, R.M., Einhorn, L.H., Loehrer, P.J. et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer 15, 1285–1291 (2007). https://doi.org/10.1007/s00520-007-0248-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-007-0248-5