Abstract
Introduction
ESD is the reference method to achieve en bloc resections for large digestive lesions. Nevertheless, it is a difficult and risky technique. Animal models exist to teach the initial skills, particularly in Japan, where pigs’ stomachs are dedicated models to gastric ESD. In Europe, we have to develop different strategies of teaching with dedicated colon models. A pig colon is a good model but thinner and narrower than a human’s. In this present work, we evaluated a bovine colon model to perform rectal ESD in retroflexion.
Methods
First, we prepared six bowels to precise the preparation protocol. Then, two endoscopists unexperienced in ESD performed 64 procedures on eight models. Learning curves and factors of variation were studied.
Results
A precise protocol to prepare the colon was defined. The two students achieved en bloc resection in 89.1 % of cases with a rate of 6.2 % of perforations. A large heterogeneity appeared between the speed and the success rate depending mainly on the age of the animal bowel. Using calf colons, the failure rates were higher (p = 0.002) and the speed was lower (p < 0.001) than for adult bovine ones. A learning curve appeared with, respectively, 0.49 and 0.59 cm2/min throughout the study. No significant difference appeared between measured and calculated specimen areas.
Discussion
Bovine colon is a new model to teach ESD in colorectal conditions. The bovine age is important to homogenize the model. A learning curve existed with a time procedure decreasing throughout the study. Further studies are needed to evaluate the precise learning curve with more students.
Conclusion
A bovine colon model is a suitable model to teach colorectal ESD. Nevertheless, an adult bovine colon model is more homogeneous than a calf one.
Similar content being viewed by others
Endoscopic submucosal dissection (ESD) appeared over one decade ago in Japan as a new method to achieve en bloc resection of superficial neoplasms without any size limit. Since then ESD has evolved greatly and shifted from the stomach to the esophagus and the colon. But, although attractive, this procedure is time consuming and risky with a significant learning curve. For beginners, the risk of perforation can reach 20 % [1–3] and it is now accepted and recommended to learn ESD on animal models before performing first human cases [4–8].
Ex vivo organs are a ready-to-use and inexpensive means to become proficient in basic ESD techniques. It is a well-documented fact that living models are more challenging in general and better adapted to learning the management of bleedings but are probably not necessary to improve initial skills. Isolated pigs’ stomachs have been the most evaluated model but are associated with a low risk of perforation because of thicker mucosal and muscular layers than humans [9–11]. Furthermore, colon lesions represent the most frequent indication for ESD in Western countries and specific tools are needed to reproduce colon conditions for trainees. In ex vivo pig colon models, the wall is thin and very fragile, which makes the procedure more challenging but not realistic. Living pig colon ESD has been studied in different studies and is now associated with high risks of perforation [12, 13]. Furthermore, pigs’ colons have very small diameter making retroflexion a difficult procedure. Bovine colon seems to be a good option as its diameter is close to the human one. Nevertheless, to the best of our knowledge, this model has never yet been used. We thus aimed to develop such a model while analyzing the factors of variation in order to homogenize it for comparative works. We then conducted a first evaluation of this model.
Methods
This study aimed to evaluate the bovine colon model to learn ESD. A first phase was defined to determine the bowel preparation protocol. Secondly, we scheduled a prospective evaluation of this model by two endoscopists unexperienced in ESD.
First phase
In this preliminary empiric work, we prepared a total of six bovine bowels to perform ESD. Ten ESDs were performed using both Dual Knife® (Olympus, Tokyo, Japan) and Nestis Enki II® (Nestis, Lyon, France). We later analyzed the problems and the advice needed to improve the model and its preparation.
Second phase
The second phase was an evaluation of the preparation protocol by two endoscopists using only the Dual Knife® (Olympus, Tokyo, Japan). For 5 days, the bowels were prepared in the morning following the preparation protocol defined previously and presented below.
Preparation protocol
We used bovine colons coming from the slaughterhouse with an agreement from the Rhône veterinary authorities to use them for a medical teaching protocol. We personally went to the slaughterhouse to prepare the colons. Both calf and adult bovines were slaughtered at the same time so we took both after identification. Definition of calf according to European regulation 700/2007 is a male or female bovine aged 8 months or less. Adult bovines used for this study were females between 12 and 24 months that had never calved (heifer) or males between 12 and 24 months. In the slaughterhouse in Lyon, calf breeds are mixed (Limousine, Montbéliard, Prim’Holstein, mixed race), but heifers and young bulls are all Limousine breed.
We received the colon from the anus and more than 1.5 m of bowel above it. We kept only the first 50 cm of the bowel above the anus, and we then carefully dissected the fat tissue surrounding the bowel. We then washed the bowel with a water jet and placed it in individual freeze bags. The anus was kept as far as possible, and we only removed it in case of perforation in the five last centimeters of the rectum that had occured during the dissection of the fat tissue. In case of holes detected during the washing above the five last centimeters, the bowel was discarded. We froze the bowels in a dedicated freezer in our animal laboratory.
The day before the procedure, around 5 pm, the bowel was taken out of the freezer and put in cold tap water. On the morning of the procedure, the bowel was filled with water to enlarge and straighten it.
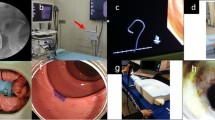
Trainees were helped by an assistant to prepare the model and to measure the resected specimens (Fig. 1). First, we inverted the bowel putting the mucosa on the external part and the serosa on the inner part. Then, the inverted bowel was inflated by air insufflation using the scope after clamping the distal edge. The assistant performed the marking of the lesions, by coagulation dots, after the inversion and the inflation of the bowel (Fig. 1A, B). Regular markings were performed by eight dots around a plastic disk of 28 mm to have a precise 30-mm lesion. Twelve lesions were drawn in the colon respecting a distance of 3 cm between two lesions. After this step, the bowel was inverted again (mucosa on the internal side) and closed by clamp on its oral side. On the anal side, it was fixed on a coelioscopic valve to avoid air leakage and then placed in the box (Fig. 1). A conduction pad surrounded by aluminum paper was placed under the bowel and then connected to the electrosurgical generator (VIO200, Erbe, Tubingen, Germany). The bowel was regularly dampened with saline solution to prevent it from drying.
Material
The knife used for the comparative study (second phase) was the Dual Knife® (Olympus, Tokyo, Japan) 1.5 mm long for all the procedures. Injection was performed with a 5-mm needle using saline solution (0.9 %) with a minute amount of indigo carmine dye. We used Olympus upper GI scope with an 8 o’clock working channel (GIF HQ-180, Olympus®, Tokyo, Japan) to which was added a distal cap attachment (4 mm length with a 3-mm side hole; D201-11304; Olympus, Tokyo, Japan). Suction was ensured with an Olympus® KV-5 pump.
Procedures
The two trainees worked on the same bowel and alternatively played the role of assistant. Before the beginning of the procedure, the trainee had to assess scope maneuver ability and retroflexed position ability. The success of the procedure was defined by a complete en bloc resection of the specimen, without perforation in <75 min. After 75 min, the procedure was stopped and considered as a failure. Complete en bloc resection was defined by the presence of the eight marking dots on a single specimen without any damage to the mucosa. In case of several pieces, the procedure was considered as a piecemeal resection and so as a failure of ESD. Perforation was carefully verified by the assistant on the bowel wall and endoscopically by the two trainees. They also counted the number of coagulation marks on the muscular layer. The volume of injection was reported for each procedure.
Specimen
The specimen size was measured at the end of the procedure on the lesion stretched on a cork piece and was defined by its two larger diameters and its surface using the ellipse formula: area (cm2) = (small diameter (cm)/2) * (big diameter (cm)/2) * π. In parallel, we also took a photograph of the piece on a scale paper to measure the area of the specimen using an Olympus® software. Dissection speed was defined as follows: speed (cm2/min) = area measured (cm2)/duration (min). In case of failure, the speed was considered as zero due to the impossibility to finish the resection within the 75 min allocated.
Endoscopists (second phase)
The two trainees were operators who currently perform EMR, EUS endoscopy and ERCP. They both had previous experience of more than 500 colonoscopies, 1,000 gastroscopies and 100 ERCPs. They had no experience of human ESD, and they both had very limited experience of ESD on animal models with no more than three cases on living animals and no more than one in ex vivo model.
Statistics
We compared the results depending on the type of bowel (calf or cow) and also between the two trainees. Quantitative variables were described using mean, standard deviation (SD) and range. The qualitative values were tabulated, and the percentages were calculated. The quantitative variables were compared using the Student’s t test or nonparametric tests (Mann–Whitney or Kruskal–Wallis tests) when appropriate. P values lower than or equal to 0.05 were considered to be statistically significant. Statistical analysis was performed with SPSS 13.0.
Results
First empiric phase
This first part was conducted as a preparation for the validation study using four bowels. Two experienced operators and one beginner prepared and worked successively to achieve a total of ten procedures on these four bowels. We used the water jet system Nestis Enki II® (Nestis, Lyon, France) in eight cases and the dual knife system in two cases during this preliminary work. Different issues were experienced and analyzed throughout this study.
Bowel preparation
We compared different thaw protocols. If the bowel was defrosted in warm water on the morning of the procedure, the submucosa was not homogenous and had different qualities of lifting depending on the site.
We also noticed different patterns on the bowel depending on the adherence around the rectum. Some of them had a very pronounced angle on the recto-sigmoid junction with increased folds in the lumen. We saw that after complete filling of the bowel with water, the adherences were broken and disappeared. Then, the bowel was straightened and the folds were similar from one bowel to another. For the first experiments, we cut the last 20 cm of the bowel which included the anus, and ESD procedures were performed on the distal colon. We experienced variation of the bowel caliber between 50 and 70 cm from the anal margin with sometimes, clear reduction in the lumen. This made ESD difficult in retroflexion. So for the second part of the study, we decided to keep the anus.
Marking dots
The first experience of marking was made after inversion of the colon but without inflation. On the flat bowel, even if we used a 30-mm disk of plastic to guide the marking, the size of the resected specimen was measured between 50 and 60 mm. This was due to the enlargement of the bowel after inflation during ESD procedures. So we adapted the protocol to do the marking after the inversion of the bowel but also with an inflation of the reversed bowel. We also used a disk of 28 mm to have a final specimen of 30 mm taking into account the size of the marking dots.
Injection
Injection was always possible using a needle or the water jet system without any issue using this model.
Difficulty
We experienced a big variation of difficulty from one bowel to another without clear evidence of the variation factor. We noticed three bowels that were difficult to dissect with procedure times over 35 min even for experienced ESD operators. Retrospectively, after the second phase of the study, we identified these three bowels as being the calf ones. One perforation occurred during procedures on a calf’s colon.
Second phase
Based on this preparation protocol, the two trainees both performed 32 procedures in 6 days alternatively. They used eight different colons (three calfs, five adults). The two trainees both performed seven procedures on calf colon and 25 on adult colons. The calf colons were the first (one procedure for each trainee), the third and the seventh bowel used.
Procedures
The main result was the ability to perform 59 complete resections (92.2 %) with 57 en bloc (89.1 %) among the 64 attempts. Two resections performed by trainee number 2 were piecemeal resections with two different pieces. The two trainees each experienced two failures by incapacity to finish the ESD procedure within the 75 min defined in the protocol. Average results are presented in Table 1. Four perforations occurred during the study (6.2 %), three with calf colons (21.4 %) and one with adult colon (2 %). This difference was not statistically significant (Fischer test, p = 0.118).
The mean procedure time was 33.3 min (range 15–75, SD ± 15.6 min) with an average dissection speed of 0.52 cm2/min (range 0–1.0, SD ± 0.2). The mean volume of saline solution injected was 105.9 ml (range 60–140, SD ± 35.8 ml).
According to the bowel type (calf or adult) (Table 2), trainees could not finish in <75 min in four procedures on calf, although it was always possible in the adult bovine’s colon. This difference of success was statistically significant with p = 0.002 (Fischer test). The average time of procedure was 54.5 min (range 32–75, SD ± 17.6) with calf versus 27.0 min with adults (range 14–43, SD ± 6.5). For dissection speed, a significant difference (p < 0.001) appeared with 0.2 cm2/min (range 0–0.7, SD ± 0.2) with calf colon versus 0.6 cm2/min (range 0.4–1.1, SD ± 0.2).
Specimens
Among the 64 procedures, 57 specimens were resected en bloc with eight marking dots. Considering the four failures, the mean maximal diameter of the 60 resected pieces was 4.9 cm (range 2.1–6, SD ± 0.7 cm) with no difference between the two trainees. The mean measured area was 15.0 cm2 (range 5.1–25.3, SD ± 3.7 cm2) with no difference between the two trainees (trainee 1: 15.7 cm2, trainee 2: 14.4 cm2). The mean calculated area using the ellipse formula was 15.6 cm2 (range 1.6–26.8, SD ± 4.6 cm2) with no difference between the two trainees (trainee 1: 16.3 cm2, trainee 2: 15.4 cm2). There was no significant difference between measured and calculated area (p = 0.460, Mann–Whitney).
Learning curve (Figs. 2, 3)
The speed of dissection increased significantly between the first half and the second half of the study (p = 0.02, Mann–Whitney test). For trainee 1, mean time and speed were, respectively, 32.2 min (range 15–75) and 0.51 cm2/min (range 0–0.93) for the first 16 procedures versus 24.3 min (14–37 min) and 0.71 cm2/min (0.38–1.11) for the last 15 cases. For trainee 2, mean time and speed were, respectively, 37.5 min (range 21–75) and 0.41 cm2/min (range 0–0.85) for the first 16 procedures versus 33.2 min (14–37 min) and 0.51 cm2/min (0.38–0.96) for the last 15 cases.
Discussion
As far as we know, this is the first report about bovine bowel isolated model to perform ESD. As reported in this study, these two skilled endoscopists with low experience in ESD could perform ESD using forward and retroflexed position with a 91.9 % rate of complete en bloc resections and a 6.2 % rate of perforations. As in other models, a progression on the dissection speed was reported in this study with a statistically significant increase from the first to the second part of the learning curve after 16 cases (p = 0.02).
Colon lesions are far more frequent than stomach neoplasia in Europe and even if it is recommended to learn ESD in the antrum in Japan [3], it is highly important to develop different teaching strategies in Western countries, using, for example, the rectum [14, 15]. In fact, colorectal ESD is riskier than gastric ESD with a risk of perforation during the first cases estimated around 35 % [16]. As described previously, the learning curve in the colon is long and challenging even for endoscopists experienced in gastric ESD [4, 17]. According to these results, there is a need to develop dedicated models to learn ESD in the colon’s specific conditions in order to reduce the risks of first procedures in humans.
The benefits of animal models were demonstrated and are now recommended as a first step to starting ESD [3, 8]. In fact, if it is possible to learn the first steps of ESD in pigs’ stomachs as described previously [18], this model is not as challenging since the mucosal and the muscular layers are very thick compared to human colons. In a pig’s stomach, the risk of perforation during early ESD experience is low compared to early experience in humans [19]. Furthermore, a new European regulation forbids our butchers to deliver complete pig stomachs without cutting them in two separate pieces. With open stomachs, we have to make a reconstruction that makes the models more complicated and it is sometimes nearly impossible to avoid air leakage. On the contrary, living pig colon is associated with very high risk of perforation, above 20 % even for experts in ESD, and it also requires animal killing. The bovine ex vivo model seems to be a good option as it is easy to find in our slaughterhouses after some administrative declarations, the preparation can be standardized and it enables to perform ESD in conditions similar to the one we encounter during human procedures.
Furthermore, we have to find anatomical conditions close to the ones we find in human rectum and colon procedures. Pig stomachs are quite different space-wise and endoscope movements are also very specific. Working in a narrow space like in a cow’s colon appears far more representative of real conditions. For this reason, keeping the anus with the distal part of the colon allows to work in a retroflexed position as in a human rectum as shown in this study.
Using this model, we observed that the age of the animals has to be known and taken into account. Indeed, ESD conditions are more homogeneous in adult colons than in calf ones. The rate of perforation was higher using calf bowels (21.4 vs. 2 %, p = 0.118), and it was often difficult to find the submucosal layer even after injection since the liquid also lifted the muscular layer. Furthermore, it was impossible to finish the procedure in 75 min in four cases using calf colons and the dissection was more difficult and slower than in adult bowels. Adult bovine colon is more similar to human mucosal conditions as a satisfactory lifting was always achieved by submucosal injections.
The bovine colon is also an evolutive training model as it is far more challenging to perform ESD in the colon above the rectosigmoid junction. This study was designed to learn rectal ESD, but we saw that it is also possible to increase the difficulty progressively by performing ESD in the upper part of the colon. Going progressively from the lower rectum to the proximal part of the colon can be interesting to improve the skills of the trainee step by step.
Our study has some limitations including the lack of randomization for calf or cow colons and the small number of procedures. As shown previously, a significant difference appeared between calf and cow colons and it seems important to take into account the animal age in order to conduct randomized studies.
In different works [11, 12], the area of the lesions is evaluated by the ellipse formula and not measured using a picture of the lesion and an area evaluation using a software. This work confirmed the good correlation between these two different methods of evaluation with no statistical difference (p = 0.460).
To summarize, the colon model is an interesting and cost-effective tool, more representative of colon conditions than a pig’s stomach and easy to prepare. A learning curve also exists with a significant increase in the dissection speed. It seems important to use bowels of adult bovines aged of 12 months and more to get conditions close to human bowels. The rate of failure using calf is high, and conditions of lifting and dissection are heterogeneous. Adult bovine are more homogenous for ESD and probably more interesting for comparative works. Measured and calculated area using ellipse formulas are not statistically different, and we can use the calculations for human trials to evaluate specimen size.
References
Parra-Blanco A (2010) Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol 16:2895
Berr F, Ponchon T, Neureiter D, Kiesslich T, Haringsma J, Kaehler GF, Schmoll F, Messmann H, Yahagi N, Oyama T (2011) Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 23:281–289
Goda K, Fujishiro M, Hirasawa K, Kakushima N, Morita Y, Oda I, Takeuchi M, Yamamoto Y, Uedo N (2012) How to teach and learn endoscopic submucosal dissection for upper gastrointestinal neoplasm in Japan. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 24(Suppl 1):136–142
Iacopini F, Bella A, Costamagna G, Gotoda T, Saito Y, Elisei W, Grossi C, Rigato P, Scozzarro A (2012) Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc 76:1188–1196
Kakushima N, Hirasawa K, Morita Y, Takeuchi M, Yamamoto Y, Oda I, Goda K, Uedo N, Fujishiro M (2012) Terminology for training of endoscopic submucosal dissection. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 24(Suppl 1):133–135
Martinek J, Suchanek S, Stefanova M, Rotnaglova B, Zavada F, Strosova A, Zavoral M (2011) Training on an ex vivo animal model improves endoscopic skills: a randomized, single-blind study. Gastrointest Endosc 74:367–373
Ohata K, Ito T, Chiba H, Tsuji Y, Matsuhashi N (2012) Effective training system in colorectal endoscopic submucosal dissection. Dig Endosc Off J Jpn Gastroenterol Endosc Soc 24(Suppl 1):84–89
Tanaka S, Morita Y, Fujita T, Wakahara C, Ikeda A, Toyonaga T, Azuma T (2012) Ex vivo pig training model for esophageal endoscopic submucosal dissection (ESD) for endoscopists with experience in gastric ESD. Surg Endosc 26:1579–1586
Parra-Blanco A, Gonzalez N, Arnau MR (2012) Ex Vivo and in vivo models for endoscopic submucosal dissection training. Clin Endosc 45:350
Parra-Blanco A, González N, González R, Ortiz-Fernández-Sordo J, Ordieres C (2013) Animal models for endoscopic training: do we really need them? Endoscopy 45:478–484
Pioche M, Ciocirlan M, Lépilliez V, Salmon D, Mais L, Guillaud O, Hervieu V, Petronio M, Lienhart I, Adriano J-L, Lafon C, Ponchon T (2014) High-pressure jet injection of viscous solutions for endoscopic submucosal dissection: a study on ex vivo pig stomachs. Surg Endosc 28:1742–1747
Ciocîrlan M, Pioche M, Lepilliez V, Gonon N, Roume R, Noel G, Pinset C, Ponchon T (2014) The ENKI-2 water-jet system versus Dual Knife for endoscopic submucosal dissection of colorectal lesions: a randomized comparative animal study. Endoscopy 46:139–143
Yahagi N, Neuhaus H, Schumacher B, Neugebauer A, Kaehler GF, Schenk M, Fischer K, Fujishiro M, Enderle MD (2009) Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy 41:340–345
Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter D (2014) Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion 89:184–193
Spychalski M, Dziki A (2014) Safe and efficient colorectal endoscopic submucosal dissection in European settings: Is successful implementation of the procedure possible? Dig Endosc. doi:10.1111/den.12353
Rahmi G, Hotayt B, Chaussade S, Lepilliez V, Giovannini M, Coumaros D, Charachon A, Cholet F, Laquière A, Samaha E, Prat F, Ponchon T, Bories E, Robaszkiewicz M, Boustière C, Cellier C (2014) Endoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in France. Endoscopy 46:670–676
Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T (2011) Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 54:1307–1312
Parra-Blanco A, Arnau MR, Nicolás-Pérez D, Gimeno-García AZ, González N, Díaz-Acosta JA, Jiménez A, Quintero E (2010) Endoscopic submucosal dissection training with pig models in a Western country. World J Gastroenterol WJG 16:2895–2900
Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D, Lepilliez V, Napoleon B, Bauret P, Coron E, Le Rhun M, Bichard P, Vaillant E, Calazel A, Bensoussan E, Bellon S, Mangialavori L, Robin F, Prat F (2011) Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy 43:664–670
Disclosures
Authors Mathieu Pioche, Jérôme Rivory, Guillermo Aguero-Garcete, Olivier Guillaud, Marc O’Brien, Cyril Lafon, Nicolas Reversat, Toshio Uraoka, Naohisa Yahagi and Thierry Ponchon have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pioche, M., Rivory, J., Aguero-Garcete, G. et al. New isolated bovine colon model dedicated to colonic ESD hands-on training: development and first evaluation. Surg Endosc 29, 3209–3215 (2015). https://doi.org/10.1007/s00464-014-4062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-4062-0