Abstract
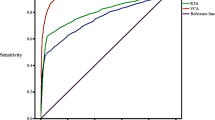
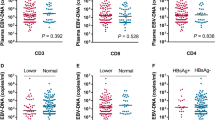
This study aimed to investigate the association between Epstein–Barr virus (EBV)-related biomarkers and TNM classification according to the seventh edition of AJCC/UICC staging system for nasopharyngeal carcinoma. Serum VCA-IgA and EA-IgA titers and plasma EBV-DNA load were quantified at baseline in 779 patients; the rates of positivity and titers/load were compared by TNM classification. The VCA-IgA-positive rate was significantly associated with advanced N classification and stage; the EA-IgA-positive rate with advanced T and N classifications and stage; the EBV-DNA-positive rate with advanced T, N and M classifications and stage. The percentage of triple-positive patients was higher in patients with advanced TNM classification. The VCA-IgA titer and EA-IgA titer correlated positively with T classification, N classification and disease stage (1:117 in Stage I, 1:188.4 in Stage II, 1:231.12 in Stage III, 1:265.91 in Stage IV, and 1:18.34 in Stage I, 1:32.11 in Stage II, 1:34.77 in Stage III, 1:37.65 in Stage IV, respectively). EBV DNA load correlated positively with T, N and M classification and stage [median lg (EBV DNA): 0 (IQ range 0–1.85) in Stage I, 1.32 (0–3.51) in Stage II, 3.33 (0–4.30) in Stage III, 3.83 (2.85–4.71) in Stage IV]. Serum VCA-IgA/EA-IgA titers and plasma EBV DNA correlated strongly with TNM classification according to the seventh edition of the AJCC/UICC; however, plasma EBV DNA load could accurately predict metastatic disease. EBV serological biomarkers may enhance the accuracy of TNM staging and help to avoid excessive imaging examinations in routine evaluation.




Similar content being viewed by others
References
Yu WM, Hussain SS (2009) Incidence of nasopharyngeal carcinoma in Chinese immigrants, compared with Chinese in China and South East Asia: review. J Laryngol Otol 123:1067–1074
Chang ET, Adami HO (2006) The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 15:1765–1777
Bensouda Y, Kaikani W, Ahbeddou N et al (2011) Treatment for metastatic nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis 128:79–85
Niedobitek G (2000) Epstein–Barr virus infection in the pathogenesis of nasopharyngeal carcinoma. Mol Pathol 53:248–254
Gu AD, Zeng MS, Qian CN (2012) The criteria to confirm the role of Epstein–Barr virus in nasopharyngeal carcinoma initiation. Int J Mol Sci 13:13737–13747
Burgos JS (2005) Involvement of the Epstein–Barr virus in the nasopharyngeal carcinoma pathogenesis. Med Oncol 22:113–121
Han BL, Xu XY, Zhang CZ et al (2012) Systematic review on Epstein–Barr virus (EBV) DNA in diagnosis of nasopharyngeal carcinoma in Asian populations. Asian Pac J Cancer Prev 13:2577–2581
Li S, Deng Y, Li X, Chen QP, Liao XC, Qin X (2010) Diagnostic value of Epstein–Barr virus capsid antigen-IgA in nasopharyngeal carcinoma: a meta-analysis. Chin Med J (Engl) 123:1201–1205
Ji MF, Wang DK, Yu YL et al (2007) Sustained elevation of Epstein–Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer 96:623–630
Chang KP, Hsu CL, Chang YL et al (2008) Complementary serum test of antibodies to Epstein–Barr virus nuclear antigen-1 and early antigen: a possible alternative for primary screening of nasopharyngeal carcinoma. Oral Oncol 44:784–792
Cai YL, Zheng YM, Wang W et al (2010) Combined detection of Epstein–Barr virus antibodies for serodiagnosis of nasopharyngeal carcinoma. Nan Fang Yi Ke Da Xue Xue Bao 30:2746–2748
Leung SF, Tam JS, Chan AT et al (2004) Improved accuracy of detection of nasopharyngeal carcinoma by combined application of circulating Epstein–Barr virus DNA and anti-Epstein–Barr viral capsid antigen IgA antibody. Clin Chem 50:339–345
Ling W, Cao SM, Huang QH, Li YH, Deng MQ (2009) Prognostic implication of pretreatment titer of serum immunoglobulin A against Epstein–Barr virus capsid antigen in nasopharyngeal carcinoma patients in Sihui, Guangdong. Ai Zheng 28:57–59
Baizig NM, Morand P, Seigneurin JM et al (2012) Complementary determination of Epstein–Barr virus DNA load and serum markers for nasopharyngeal carcinoma screening and early detection in individuals at risk in Tunisia. Eur Arch Otorhinolaryngol 269:1005–1011
Chai SJ, Pua KC, Saleh A et al (2012) Clinical significance of plasma Epstein–Barr virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J Clin Virol 55:34–39
Lin JC, Wang WY, Chen KY et al (2004) Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 350:2461–2470
Hou X, Zhang L, Zhao C et al (2006) Prognostic impact of plasma Epstein–Barr virus DNA concentration on distant metastasis in nasopharyngeal carcinoma. Ai Zheng 25:785–792
Leung SF, Chan AT, Zee B et al (2003) Pretherapy quantitative measurement of circulating Epstein–Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 98:288–291
Sun R, Qiu HZ, Mai HQ et al (2013) Prognostic value and differences of the sixth and seventh editions of the UICC/AJCC staging systems in nasopharyngeal carcinoma. J Cancer Res Clin Oncol 139:307–314
Chen L, Mao YP, Xie FY et al (2012) The seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma is prognostically useful for patients treated with intensity-modulated radiotherapy from an endemic area in China. Radiother Oncol 104:331–337
Lo YM, Leung SF, Chan LY et al (2000) Plasma cell-free Epstein–Barr virus DNA quantitation in patients with nasopharyngeal carcinoma. Correlation with clinical staging. Ann N Y Acad Sci 906:99–101
Shao JY, Li YH, Gao HY et al (2004) Comparison of plasma Epstein–Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 100:1162–1170
Cai YL, Zheng YM, Cheng JR et al (2010) Relationship between clinical stages of nasopharyngeal carcinoma and Epstein–Barr virus antibodies Rta/IgG, EBNA1/IgA, VCA/IgA and EA/IgA. Nan Fang Yi Ke Da Xue Xue Bao 30:509–511
Zeng Y, Zhang LG, Wu YC et al (1985) Prospective studies on nasopharyngeal carcinoma in Epstein–Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int J Cancer 36:545–547
Chan KC, Hung EC, Woo JK et al (2013) Early detection of nasopharyngeal carcinoma by plasma Epstein–Barr virus DNA analysis in a surveillance program. Cancer 119:1838–1844
Leung SF, Zee B, Ma BB et al (2006) Plasma Epstein–Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 24:5414–5418
Xu J, Wan XB, Huang XF et al (2010) Serologic antienzyme rate of Epstein–Barr virus DNase-specific neutralizing antibody segregates TNM classification in nasopharyngeal carcinoma. J Clin Oncol 28:5202–5209
Wang WY, Twu CW, Chen HH et al (2013) Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein–Barr virus DNA levels. Cancer 119:963–970
An X, Wang FH, Ding PR et al (2011) Plasma Epstein–Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer 117:3750–3757
Hsu CL, Chang KP, Lin CY et al (2012) Plasma Epstein–Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck 34:1064–1070
Chan AT (2010) Nasopharyngeal carcinoma. Ann Oncol 21:308–312
National Comprehensive Cancer Network (2013) NCCN clinical practice guidelines in oncology for head and neck cancer, Version 2. http://www.nccn.org/index.asp
Tang LQ, Chen QY, Fan W et al (2013) Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein–Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 31:2681–2689
Acknowledgments
This study was supported by grants from the General Funds of the National Natural Science Foundation of China (No. 81272575) and the Science and Technology Planning Project of Guangdong Province, China (Nos. 2010B080701015 and 2012A030400038).
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author information
Authors and Affiliations
Corresponding authors
Additional information
P. Sun and C. Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sun, P., Chen, C., Cheng, YK. et al. Serologic biomarkers of Epstein–Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 271, 2545–2554 (2014). https://doi.org/10.1007/s00405-013-2805-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-013-2805-5