Abstract
Purpose
Previous epidemiological studies on egg consumption and the risk of gastrointestinal (GI) neoplasms suggest a positive association; however, data are limited and the evidence remains controversial. This study aims to investigate and quantify the potential dose–response relationship with an evaluation of cancer site-specific differences.
Methods
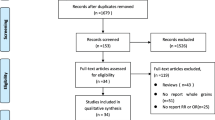
Relevant studies were identified after the literature search via electronic databases until January 2014. Subgroup analysis for serving portions was performed using two standardized classification methods: (1) less than 3, or 3 or more eggs per week; (2) less than 3, 3–5, or more than 5 eggs per week. Method two excludes studies that only reported consumption frequency. Pooled adjusted odds ratios (ORs) comparing highest and lowest categories of dietary pattern scores were calculated using a random-effects model.
Results
Thirty-seven case–control and seven cohort studies were included for meta-analysis, which contained a total of 424,867 participants and 18,852 GI neoplasm cases. The combined odds ratio (OR) was calculated to 1.15 (95 % CI 1.09–1.22; p value heterogeneity <0.001), showing only a slight increase in risk. The correlation was stronger for colon cancers 1.29 (95 % CI 1.14–1.46; p value heterogeneity <0.22). Dose–response analysis revealed similar results with stratification methods, and the ORs for an intake of <3 and ≥3 eggs per week were 1.14 (95 % CI 1.07–1.22; p value heterogeneity = 0.38) and 1.25 (95 % CI 1.14–1.38; p value heterogeneity = 0.25), respectively. With method 2, the ORs for an intake of <3, 3–5, and >5 eggs per week were 1.13 (95 % CI 1.06–1.21; p value heterogeneity = 0.25), 1.14 (95 % CI 1.01–1.29; p value heterogeneity = 0.06), and 1.19 (95 % CI 1.01–1.39; p value heterogeneity <0.001), respectively.
Conclusion
This study provides evidence that egg consumption is associated with a positive dose–response association with the development of GI neoplasms.


Similar content being viewed by others
References
World Cancer Research Fund (2007) Food, nutrition, physical activity and the prevention of cancer: a global perspective. American Institute for Cancer Research, Washington, DC, USA
Steinmetz KA, Potter JD (1994) Egg consumption and cancer of the colon and rectum. Eur J Cancer Prev 3:237–245
Gonzalez CA, Sanz JM, Marcos G, Pita S, Brullet E, Saigi E, Badia A, Riboli E (1991) Dietary factors and stomach cancer in Spain: a multi-centre case-control study. Int J Cancer 49:513–519
Gao CM, Takezaki T, Ding JH, Li MS, Tajima K (1999) Protective effect of allium vegetables against both esophageal and stomach cancer: a simultaneous case-referent study of a high-epidemic area in Jiangsu Province, China. Jpn J Cancer Res 90:614–621
Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, Hanaoka T, Tsugane S, Sao Paulo-Japan Cancer Project Gastric Cancer Study G (2002) Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in Sao Paulo. Jpn J Clin Oncol 32:277–283
Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S (1999) Food groups and colorectal cancer risk. Br J Cancer 79:1283–1287
Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S (2000) Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer 87:289–294
Block G, Dresser CM, Hartman AM, Carroll MD (1985) Nutrient sources in the American diet: quantitative data from the NHANES II survey. II. Macronutrients and fats. Am J Epidemiol 122:27–40
Cruse P, Lewin M, Clark CG (1979) Dietary cholesterol is co-carcinogenic for human colon cancer. Lancet 1:752–755
Sakaguchi M, Minoura T, Hiramatsu Y, Takada H, Yamamura M, Hioki K, Yamamoto M (1986) Effects of dietary saturated and unsaturated fatty acids on fecal bile acids and colon carcinogenesis induced by azoxymethane in rats. Cancer Res 46:61–65
Bidoli E, Franceschi S, Talamini R, Barra S, La Vecchia C (1992) Food consumption and cancer of the colon and rectum in north-eastern Italy. Int J Cancer 50:223–229
Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H (2005) Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 589:47–65
Cho E, Willett WC, Colditz GA, Fuchs CS, Wu K, Chan AT, Zeisel SH, Giovannucci EL (2007) Dietary choline and betaine and the risk of distal colorectal adenoma in women. J Natl Cancer Inst 99:1224–1231
Zeisel SH, Mar MH, Howe JC, Holden JM (2003) Concentrations of choline-containing compounds and betaine in common foods. J Nutr 133:1302–1307
Chandar N, Lombardi B (1988) Liver cell proliferation and incidence of hepatocellular carcinomas in rats fed consecutively a choline-devoid and a choline-supplemented diet. Carcinogenesis 9:259–263
Nakagami K, Uchida T, Ohwada S, Koibuchi Y, Morishita Y (1999) Increased choline kinase activity in 1,2-dimethylhydrazine-induced rat colon cancer. Jpn J Cancer Res 90:1212–1217
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB, Grp M (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA 283:2008–2012
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. doi:10.1136/bmj.327.7414.557
Egger M, Smith GD, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Kampman E, Verhoeven D, Sloots L, van’t Veer P (1995) Vegetable and animal products as determinants of colon cancer risk in Dutch men and women. Cancer Causes Control 6:225–234
Matthew JA, Johnson IT (1995) Egg consumption and risk-markers for colorectal neoplasia. Eur J Cancer Prev 4:425–428
Yoon H, Benamouzig R, Little J, Francois-Collange M, Tome D (2000) Systematic review of epidemiological studies on meat, dairy products and egg consumption and risk of colorectal adenomas. Eur J Cancer Prev 9:151–164
Hiramatsu Y, Takada H, Yamamura M, Hioki K, Saito K, Yamamoto M (1983) Effect of dietary cholesterol on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis 4:553–558
Song WO, Kerver JM (2000) Nutritional contribution of eggs to American diets. J Am Coll Nutr 19:556S–562S
Morin RJ, Hu B, Peng SK, Sevanian A (1991) Cholesterol oxides and carcinogenesis. J Clin Lab Anal 5:219–225
Nagengast FM, Grubben MJ, van Munster IP (1995) Role of bile acids in colorectal carcinogenesis. Eur J Cancer 31A:1067–1070
Lin TM (1975) Actions of gastrointestinal hormones and related peptides on the motor function of the biliary tract. Gastroenterology 69:1006–1022
Bjeldanes LF, Morris MM, Felton JS, Healy S, Stuermer D, Berry P, Timourian H, Hatch FT (1982) Mutagens from the cooking of food. II. Survey by Ames/salmonella test of mutagen formation in the major protein-rich foods of the American diet. Food Chem Toxicol 20:357–363
Zheng W, Lee SA (2009) Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer 61:437–446
Le Marchand L, Wilkens LR, Hankin JH, Kolonel LN, Lyu LC (1997) A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): lipids and foods of animal origin. Cancer Causes Control 8:637–648
Aune D, De Stefani E, Ronco AL, Boffetta P, Deneo-Pellegrini H, Acosta G, Mendilaharsu M (2009) Egg consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev 10:869–876
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tse, G., Eslick, G.D. Egg consumption and risk of GI neoplasms: dose–response meta-analysis and systematic review. Eur J Nutr 53, 1581–1590 (2014). https://doi.org/10.1007/s00394-014-0664-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-014-0664-5