Abstract
Purpose
Treatment options for patients with platinum-refractory recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) are limited. The purpose of this study was to assess the efficacy and safety of zalutumumab in platinum-refractory R/M SCCHN.
Methods
Patients with platinum-refractory R/M SCCHN were enrolled if they had performance status of 0–2, age ≥18 years and adequate organ function. Patients received weekly infusions of zalutumumab individually titrated to a grade 2 skin rash. Primary objective was overall survival (OS), and secondary objectives were efficacy and safety. A subgroup analysis of OS and progression-free survival (PFS) was conducted for various demographic, disease-related and molecular factors.
Results
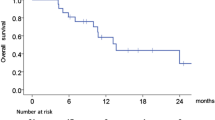
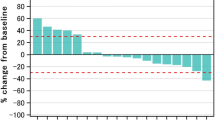
Ninety patients were enrolled. Twenty-three percent of patients had performance status (PS) 2 and 74 % had distant metastases. Median OS was 5.3 months (95 % CI [4.1, 7.1]), and median PFS was 2.1 months (95 % CI [2.0, 2.6]). Subgroup analysis by ECOG PS revealed median OS of 6.3 months for PS = 0–1 and 2.5 months for PS = 2. Objective response rate was 5.7 %, and disease control rate was 39.8 %. Grade 3–4 adverse events related to zalutumumab were observed in 19 % of patients and included skin rash (5 %), hypomagnesemia (4 %) and pneumonitis (1 %). The frequency of all-cause grade 3–4 AEs was 62 % and included infections (14 %), gastrointestinal disorders (12 %) and hypokalemia (6 %). Two deaths were deemed related to zalutumumab [ClinicalTrials.gov Identifier: NCT00542308].
Conclusions
Zalutumumab showed reasonable efficacy in platinum-refractory R/M SCCHN patients, and dose titration based on skin rash evaluation was feasible.





Similar content being viewed by others
References
Ferlay J, Bray F, Pisani P, Parkin DM (2004) Globocan 2002: cancer incidence, mortality and prevalence worldwide. IARC Cancerbase No. 5. Version 2.0. IARCPress, Lyon
Vermorken JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. M Engl J Med 359:1116–1127
Leon X, Hitt R, Constenla M et al (2005) A retrospective analysis of the outcome of patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck refractory to a platinum-based chemotherapy. Clin Oncol R Coll Radiol 17:418–424
Vermorken JB, Trigo J, Hitt R et al (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25(16):2171–2177
Ang KK, Berkey BA, Tu X et al (2002) Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 62:7350–7356
Grandis JR, Melhem MF, Gooding WE et al (1998) Levels of TGF-alpha and EGFr protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90:824–832
Sheikh Ali MA, Gunduz M, Nagatsuka H et al (2008) Expression and mutation analysis of epidermal growth factor receptor in head and neck squamous cell carcinoma. Cancer Sci 99:1589–1594
Wei Q, Sheng L, Shui Y, Hu Q, Nordgren H, Carlsson J (2008) EGFr, HER2, and HER3 expression in laryngeal primary tumors and corresponding metastases. Ann Surg Oncol 15:1193–1201
van Bueren JJL, Bleeker WK, Brannstrom A et al (2008) The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility. Proc Natl Acad Sci USA 105:6109–6114
Bleeker WK, van Bueren JJL et al (2004) Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol 173:4699–4707
Bastholt L, Jensen K et al (2007) Phase I/II clinical and pharmacokinetic study evaluating a fully human monoclonal antibody against EGFr (HuMax-EGFr) in patients with advanced squamous cell carcinoma of the head and neck. Radiother Oncol 85(1):24–28
Machiels JP, Subramanian S, Ruzsa A et al (2011) Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol 12(4):333–343
Peeters M, Siena S, Van Cutsem E et al (2009) Association of progression-free survival, overall survival and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer 115:1544–1554
Tejpar S, Peeters M, Humblet Y et al (2007) Phase I/II study of cetuximab dose-escalation in patients with metastatic colorectal cancer (mCRC) with no or slight skin reaction on cetuximab standard dose treatment (EVEREST): pharmacokinetic (PK), pharmacodynamics (PD) and efficacy data. Proc Am Soc Clin Oncol 24:3554
Perez-Soler R, Saltz L (2005) Cutaneous adverse effects with HER1/EGFr-targeted agents: is there a silver lining? J Clin Oncol 23:5235–5246
Saltz L, Rubin MS, Hochster H (2001) Acne-like rash predicts response in patients with cetuximab (IMC-225) plus irinotecan (CPT-11) in CPT-11-refractory colorectal cancer (CRC) that expresses epidermal growth factor receptor (EGFr). Clin Cancer Res 7:3766
Bonner JA, Jarari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomized trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11:11–28
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB et al (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Cohen MH, Williams GA, Sridhara R et al (2004) United States Food and Drug Administration drug approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res 10:1212–1218
Camus P, Kudoh S, Ebina M (2004) Interstitial lung disease associated with drug therapy. Br J Cancer 91(suppl 2):S18–S23
Ando M, Okamoto I, Yamamoto N, Takeda K, Tamura K, Seto T, Ariyoshi Y, Fukuoka M (2006) Predictive factors for interstitial lung disease, antitumor response, and survival in non-small-cell lung cancer patients treated with gefitinib. J Clin Oncol 24(16):2549–2556
Ratushny V, Astsaturov I, Burtness BA, Golemis EA, Silverman JS (2009) Targeting EGFR resistance networks in head and neck cancer. Cell Signal 21(8):1255–1268
Mehra R, Serebriiskii IG, Dunbrack RL Jr, Robinson MK, Burtness B, Golemis EA (2011) Protein-intrinsic and signaling network-based sources of resistance to EGFR- and ErbB family-targeted therapies in head and neck cancer. Drug Resist Updat 14(6):260–279
Young NR, Liu J, Pierce C, et al (2012) Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition. Mol Oncol. doi:10.1016/j.molonc.2012.11.001
Chen LF, Cohen EE, Grandis JR (2010) New strategies in head and neck cancer: understanding resistance to epidermal growth factor receptor inhibitors. Clin Cancer Res 16(9):2489–2495
Acknowledgments
The independent data monitoring committee consisted of Dr. Jens Benn-Sørensen (Department of Oncology, The Finsen Centre, Rigshospitalet, National University Hospital, Denmark), Dr. med. Torkil Menné (Department of Dermatology, KAS-Gentofte, Denmark), and Dr Barbara Ann Burtness (Fox Chase Cancer Center, USA). We thank the patients and the following investigators in the Hx-EGFR-205 Study Group for their participation in the study: Austria; Dr. Gabriela Veran Kornek (Vienna); Czech Republik; Dr. Petra Holečková (Prague), Dr. Hana Honova (Prague), Dr. Lubomir Slavicek (Jihlava); Germany; Dr. Gerhard Dyckhoff (Heidelberg), Dr. Markus Hambek (Frankfurt), Dr. Jens Büntzel (Nordhausen); Israel; Dr. Rami Ben-Yosef (Tel-Aviv), Dr. Raphael Pfeffer (Ramat-Gan), Dr. Tal Grenader (Jerusalem), Dr. Konstantin Lavrenkov (Beer Shiva); Italy; Dr. Franco Nolé (Milan); Portugal; Dr. Isabel Goulão Sargento (Lisboa), Dr. Isabel Pazos Portela (Coimbra); Slovak Republik; Dr. Ludovit Jurga (Trnava), Dr. Tomas Minarik (Bratislava); Chile; Dr. Eduardo Patricio Yañez Ruiz (Temuco), Dr. Pablo Gonzalez Mella (Vina del Mar), Dr. Jose Antonio Solis Campos (Valparaiso); Colombia; Dr. Manuel Enrique Gonzalez (Monteria); Peru; Dr. Julio Santiago Guevara Guevara (Lima), Dr. Lucila Regina Morla Chiong Kongfook (Lima), Dr. Diana Sofia Aleman Polanco (Arequipa); USA; Eben Rosenthal (Birmingham, AL), Tawee Tanvetyanon (Tampla, FL), Dr. Tanguy Seiwert (Chicago, IL), Sreenivasa Nattam (Fort Wayne, IN), Neil Senzer (Dallas, TX), Neil Gross (Portland, OR), Mary Jo Fidler (Chicago, IL), Theodore Walters (Boise, ID), Chung-Tsen Hsueh (Loma Linda, CA), Lucio Gordan (Gainsville, FL). This study was designed and funded by Genmab, who also had oversight over the study, safety reporting, data collection and assembly as well as data interpretation.
Conflict of interest
VS has no conflicts of interest. EC has consultant role for Boehringer Ingelheim, Novartis, Genmab, Bristol Myers Squibb, AstraZeneca and Exelixis. LL has advisory/consultant role for BMS, Glaxo, Lilly, Merck-Serono, Amgen, Boehringer Ingelheim and Pfizer. JD has advisory/consultant role for Amgen and Merck-Serono and has received honoraria from Merck-Serono. SL is senior medical director of Genmab and holds stock options. TG has advisory/consultant role for Amgen, Boehringer Ingelheim, Genmab, Merck-Serono and Novartis and has received honoraria from Amgen, Boehringer Ingelheim and Merck-Serono.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saloura, V., Cohen, E.E.W., Licitra, L. et al. An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 73, 1227–1239 (2014). https://doi.org/10.1007/s00280-014-2459-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2459-z