Abstract
Objective
We investigated whether aprepitant, a neurokinin-1 antagonist, could decrease chemotherapy-induced nausea and vomiting (CINV) following cisplatin, when a conventional anti-emetic regimen had failed.
Methods
This was a prospective study (April 2011–April 2012) of patients with lung cancer, treated with cisplatin at the Beijing Cancer Hospital, and initially receiving granisetron, dexamethasone, and metoclopramide as anti-emetics. If patients experienced vomiting of grade ≥2 and required rescue anti-emetic medications during the first cycle, oral aprepitant was added in subsequent cycles (day 1: 125 mg; days 2–3: 80 mg once daily). Acute (day 1) and delayed (days 2–5) nausea and vomiting, use of rescue medications, and occurrence of adverse reactions were monitored after the start of chemotherapy.
Results
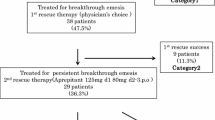
Twenty-five of 132 patients (18.9 %) were administered aprepitant for secondary prophylaxis against emesis during the second cycle of chemotherapy. The incidences of acute and delayed nausea were 52 and 100 % in the first cycle, but 8 and 72 % in the second cycle. The incidences of acute and delayed vomiting were 20 and 100 % in the first cycle, but 0 and 36 % in the second cycle. No patients required rescue medications or intravenous rehydration during the second cycle. Aprepitant was not associated with additional adverse events.
Conclusions
In patients with lung cancer receiving cisplatin-based chemotherapy, the addition of aprepitant to a 5-HT3 antagonist, dexamethasone, and metoclopramide improves protection against CINV when the conventional anti-emetic regimen fails.


Similar content being viewed by others
References
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. N Engl J Med 358(23):2482–2494. doi:10.1056/NEJMra0706547
Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D, Group EMGW (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243. doi:10.1093/annonc/mdq194
Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, Somerfield MR, Chesney M, Clark-Snow RA, Flaherty AM, Freundlich B, Morrow G, Rao KV, Schwartz RN, Lyman GH, American Society of Clinical Oncology (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29(31):4189–4198. doi:10.1200/JCO.2010.34.4614
Hesketh PJ (1999) Defining the emetogenicity of cancer chemotherapy regimens: relevance to clinical practice. Oncologist 4(3):191–196
Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol 17(9):2971–2994
Roila F, Tonato M, Cognetti F, Cortesi E, Favalli G, Marangolo M, Amadori D, Bella MA, Gramazio V, Donati D et al (1991) Prevention of cisplatin-induced emesis: a double-blind multicenter randomized crossover study comparing ondansetron and ondansetron plus dexamethasone. J Clin Oncol 9(4):675–678
Smith DB, Newlands ES, Rustin GJ, Begent RH, Howells N, McQuade B, Bagshawe KD (1991) Comparison of ondansetron and ondansetron plus dexamethasone as antiemetic prophylaxis during cisplatin-containing chemotherapy. Lancet 338(8765):487–490
Verweij J, de Wit R, de Mulder PH (1996) Optimal control of acute cisplatin-induced emesis. Oncology 53(Suppl 1):56–64
Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, Sawyer WT (1999) Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract 7(2):59–65
Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H (2007) Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 15(5):497–503. doi:10.1007/s00520-006-0173-z
Laszlo J, Lucas VS Jr (1981) Emesis as a critical problem in chemotherapy. N Engl J Med 305(16):948–949. doi:10.1056/NEJM198110153051609
Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, Suarez C, Zorrilla I, Gomez J, Zabaleta P, Nocea G, Llombart-Cussac A (2012) Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer 20(12):3141–3148. doi:10.1007/s00520-012-1448-1
Diemunsch P, Grelot L (2000) Potential of substance P antagonists as antiemetics. Drugs 60(3):533–546
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F, Aprepitant Protocol 054 Study Group (2003) Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97(12):3090–3098. doi:10.1002/cncr.11433
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ (2003) The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin–the Aprepitant Protocol 052 Study Group. J Clin Oncol 21(22):4112–4119. doi:10.1200/JCO.2003.01.095
de Wit R, Herrstedt J, Rapoport B, Carides AD, Carides G, Elmer M, Schmidt C, Evans JK, Horgan KJ (2003) Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 21(22):4105–4111. doi:10.1200/JCO.2003.10.128
Herrstedt J, Muss HB, Warr DG, Hesketh PJ, Eisenberg PD, Raftopoulos H, Grunberg SM, Gabriel M, Rodgers A, Hustad CM, Horgan KJ, Skobieranda F, Aprepitant Moderately Emetogenic Chemotherapy Study Group (2005) Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer 104(7):1548–1555. doi:10.1002/cncr.21343
Rojas C, Li Y, Zhang J, Stathis M, Alt J, Thomas AG, Cantoreggi S, Sebastiani S, Pietra C, Slusher BS (2010) The antiemetic 5-HT3 receptor antagonist Palonosetron inhibits substance P-mediated responses in vitro and in vivo. J Pharmacol Exp Ther 335(2):362–368. doi:10.1124/jpet.110.166181
Hesketh PJ, Younger J, Sanz-Altamira P, Hayden M, Bushey J, Trainor B, Krentzin M, Nowd P, Arnaoutakis K, Hesketh AM (2009) Aprepitant as salvage antiemetic therapy in breast cancer patients receiving doxorubicin and cyclophosphamide. Support Care Cancer 17(8):1065–1070. doi:10.1007/s00520-008-0545-7
Abbrederis K, Lorenzen S, Rothling N, Ihbe-Heffinger A, Schuster T, Peschel C, Lordick F (2009) Chemotherapy-induced nausea and vomiting in the treatment of gastrointestinal tumors and secondary prophylaxis with aprepitant. Onkologie 32(1–2):30–34. doi:10.1159/000183735
Oechsle K, Müller MR, Hartmann JT, Kanz L, Bokemeyer C (2006) Aprepitant as salvage therapy in patients with chemotherapy-induced nausea and emesis refractory to prophylaxis with 5-HT(3) antagonists and dexamethasone. Onkologie 29(12):557–561
Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, Cruciani G, Daniele B, De Pouvourville G, Rubenstein EB, Daugaard G (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100(10):2261–2268. doi:10.1002/cncr.20230
Roila F (2000) Prevention of cisplatin-induced delayed emesis: still unsatisfactory. Italian Group for Antiemetic Research. Support Care Cancer 8(3):229-232
Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR (2003) Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: a University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer 97(11):2880–2886. doi:10.1002/cncr.11408
Glaus A, Knipping C, Morant R, Bohme C, Lebert B, Beldermann F, Glawogger B, Ortega PF, Husler A, Deuson R (2004) Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer 12(10):708–715. doi:10.1007/s00520-004-0662-x
Navari RM, Reinhardt RR, Gralla RJ, Kris MG, Hesketh PJ, Khojasteh A, Kindler H, Grote TH, Pendergrass K, Grunberg SM, Carides AD, Gertz BJ (1999) Reduction of cisplatin-induced emesis by a selective neurokinin-1-receptor antagonist. L-754,030 Antiemetic Trials Group. N Engl J Med 340(3):190–195. doi:10.1056/NEJM199901213400304
Campos D, Pereira JR, Reinhardt RR, Carracedo C, Poli S, Vogel C, Martinez-Cedillo J, Erazo A, Wittreich J, Eriksson LO, Carides AD, Gertz BJ (2001) Prevention of cisplatin-induced emesis by the oral neurokinin-1 antagonist, MK-869, in combination with granisetron and dexamethasone or with dexamethasone alone. J Clin Oncol 19(6):1759–1767
Yeo W, Mo FK, Suen JJ, Ho WM, Chan SL, Lau W, Koh J, Yeung WK, Kwan WH, Lee KK, Mok TS, Poon AN, Lam KC, Hui EK, Zee B (2009) A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Cancer Res Treat 113(3):529–535. doi:10.1007/s10549-008-9957-9
Vardy J, Chiew KS, Galica J, Pond GR, Tannock IF (2006) Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer 94(7):1011–1015. doi:10.1038/sj.bjc.6603048
Jin Y, Wu X, Guan Y, Gu D, Shen Y, Xu Z, Wei X, Chen J (2012) Efficacy and safety of aprepitant in the prevention of chemotherapy-induced nausea and vomiting: a pooled analysis. Support Care Cancer 20(8):1815–1822. doi:10.1007/s00520-011-1280-z
Conflict of interest
The authors declare that there are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, W., Fang, J., Nie, J. et al. Addition of aprepitant improves protection against cisplatin-induced emesis when a conventional anti-emetic regimen fails. Cancer Chemother Pharmacol 73, 1129–1136 (2014). https://doi.org/10.1007/s00280-014-2446-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2446-4