Abstract
Purpose
The mammalian target of rapamycin complex 1 (mTORC1) is aberrantly activated in many head and neck squamous cell carcinomas (HNSCCs). This phase I study combines the mTORC1 inhibitor temsirolimus with carboplatin and paclitaxel.
Methods
This was a single institution phase I study for patients with R/M HNSCC with a standard 3 + 3 design. Three doses of temsirolimus were planned: 15, 20, and 25 mg. Due to excessive toxicity with the original study regimen, the protocol was amended to carboplatin AUC 1.5, paclitaxel 80 mg/m2, and temsirolimus (according to dose escalation plan), all on days 1 and 8 of a 21-day cycle.
Results
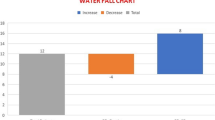
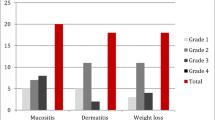
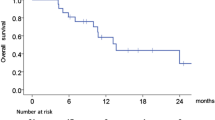
18 patients (14 male, 4 female) enrolled, with median age 56 years (range 33–78). The most common toxicities were anemia, leukopenia, thrombocytopenia, and hyperglycemia. Among all patients treated, the confirmed objective partial response (cPR) rate was 22 %. DLT was not exceeded among 6 patients treated at dose level 3 of the revised protocol, and 4 of 6 subjects treated at this dose level had cPRs.
Conclusion
The phase II recommended regimen is temsirolimus 25 mg, carboplatin AUC 1.5, and paclitaxel 80 mg/m2, all on days 1 and 8 of a 21-day cycle. A phase II study of this regimen in R/M HNSCC is ongoing.
Similar content being viewed by others
References
Aissat N, Le Tourneau C, Ghoul A, Serova M, Bieche I, Lokiec F, Raymond E, Faivre S (2008) Antiproliferative effects of rapamycin as a single agent and in combination with carboplatin and paclitaxel in head and neck cancer cell lines. Cancer Chemother Pharmacol 62:305–313
Amornphimoltham P, Patel V, Sodhi A, Nikitakis NG, Sauk JJ, Sausville EA, Molinolo AA, Gutkind JS (2005) Mammalian target of rapamycin, a molecular target in squamous cell carcinomas of the head and neck. Cancer Res 65:9953–9961
Andre F, Campone M, O’Regan R, Manlius C, Massacesi C, Sahmoud T, Mukhopadhyay S, Soria J-C, Naughton M, Hurvitz SA (2010) Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol 28:5110–5115
Belani CP, Ramalingam S, Perry MC, LaRocca RV, Rinaldi D, Gable PS, Tester WJ (2008) Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 26:468–473
Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly E, Natt F, Hall J, Lane HA, Thomas G (2005) The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damage induced apoptosis though inhibition of p21 translation. Cell 120:747–759
Campone M, Levy V, Bourbouloux E, Berton Riguad D, Bootle D, Dutreix C, Zoellner U, Shand N, Calvo F, Raymond E (2009) Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumors. Br J Cancer 100:315–321
Faried LS, Faried A, Kanuma T, Nakazato T, Tamura T, Kuwano H, Minegishi T (2006) Inhibition of the mammalian target of rapamycin (mTOR) by rapamycin increases chemosenstivity of CaSki cells to paclitaxel. Eur J Cancer 42:934–947
Figlin RA, Brown E, Armstrong AJ, Akerley W, Benson AB, Burstein HJ, Ettinger DS, Febbo PG, Fury MG, Hudes GR, Kies MS, Kwak EL, Morgan RJ, Mortimer J, Reckamp K, Venook AP, Worden F, Yen Y (2008) NCCN Task Force Report: mTOR inhibition in solid tumors. J Natl Compr Canc Netw 6(Suppl 5):S1–S22
Forastiere A, Shank D, Neuberg D, Taylor SG, DeConti RC, Adams G (1998) Final report of a phase II evaluation of paclitaxel in patients with advanced squamous cell carcinoma of the head and neck: an Eastern Cooperative Oncology Group Trial (PA390). Cancer 82:2270–2274
Fury MG, Pfister DG (2011) Current recommendations for systemic therapy of recurrent and/or metastatic head and neck squamous cell cancer. J Natl Compr Canc Netw 9:681–689
Fury MG, Sherman E, Haque S, Korte S, Lisa D, Shen R, Wu N, Pfister DG (2012) A phase I study of everolimus plus low-dose weekly cisplatin for patients with advanced solid tumors. Cancer Chemother Pharmacol 69:591–598
Harding MW (2003) Immunophilins, mTOR, and pharmacodynamic strategies for a targeted cancer therapy. Clin Cancer Res 9:2882–2886
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IGH, Barbarash O, Gokmen E, O’Toole T, Lustarten S, Moore L, Motzer RJ (2007) Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. New Engl J Med 356:2271–2281
Kollmannsberger C, Hirte H, Siu LL, Mazurka J, Chi K, Elit L, Walsh W, Sedarias J, Doyle A, Eisenhauer EA, Oza AM (2012) Temsirolimus in combination with carboplatin and paclitaxel in patients with advanced solid tumors: a NCIC-CTG, phase 1, open-label dose-escalation study (IND 179). Ann Oncol 23:238–244
Molinolo AA, Hewitt SM, Amornphimoltham P, Keelewat S, Rangdaeng S, Garcia AM, Raimondi AR, Jufe R, Itoiz M, Gao Y, Saranath D, Kaleebi GS, Yoo GH, Leak L, Myers EM, Shintani S, Wong D, Massey HD, Yeudall WA, Lonardo F, Ensley J, Gutkind JS (2007) Dissecting the Akt/mammalian target of rapamycin signaling network: emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res 13:4964–4973
Mondesire WH, Jian W, Zhang H, Ensor J, Hung M-C, Mills GB, Meric-Bernstam F (2004) Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin Cancer Res 10:7031–7042
Moulder S, Gladish G, Ensor J, Gonzalez-Angulo AM, Cristofanillo M, Murray JM, Booser D, Giordano SH, Brewster A, Moore J, Rivera E, Hortobagyi GN, Tran HT (2011) A phase 1 study of weekly everolimus (RAD001) in combination with docetaxel in patients with metastatic breast cancer. Cancer [Epub ahead of print]
Nathan C-AO, Amirghahari N, Rong X, Giordano T, Sibley D, Nordberg M, Glass J, Agarwal A, Caldito G (2007) Mammalian target of rapamycin inhibitors as possible adjuvant therapy for microscopic residual disease in head and neck squamous cell cancer. Cancer Res 67:2160–2168
Oza AM, Kollmannsberger C, Hirte H, Welch S, Siu L, Mazurka J, Sedarias J, Doyle LA, Eisenhauer E (2009) Phase I study of temsirolimus (CCI-779), carboplatin, and paclitaxel in patients with advanced solid tumors: NCI CTG IND 179. J Clin Oncol 27:15s (suppl; abstr 3558)
Perez EA, Suman VJ, Rowland KM, Ingle JN, Salim M, Loprinzi CL, Flynn PJ, Mailliard JA, Kardinal CG, Krook JE, Thrower AR, Visscher DW, Jenkins RB (2005) Two concurrent phase II trials of paclitaxel/carboplatin/trastuzumab (weekly or every-three-week schedule) as first-line therapy in women with HER-2 expressing metastatic breast cancer: NCCTG study 983252. Clin Breast Cancer 6:425–432
Ramalingam SS, Harvey D, Saba N, Owonikoko TK, Kauh J, Shin DM, Sun S-Y, Strychor S, Tighiouart M, Egorin M, Fu H, Khuri FR (2010) Phase I and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel in recurrent/refractory nonsmall cell lung cancer. Cancer 116:3903–3909
Shi Y, Frankel A, Radvanyi LG, Penn LZ, Miller RG, Mills GB (1995) Rapamycin enhances apoptosis and increases sensitivity to cisplatin in vitro. Cancer Res 55:1982–1988
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer H-R, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Racourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. New Engl J Med 359:1116–1127
Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, Knecht R, Amella N, Schueler A, Baselga J (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based chemotherapy. J Clin Oncol 25:2171–2177
Wangpaichitr M, Wu C, You M, Kuo MT, Feun L, Lampidis T, Savaraj N (2008) Inhibition of mTOR restores cisplatin sensitivity through down-regulation of growth and anti-apoptotic proteins. Eur J Pharm 591:124–127
Acknowledgments
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) from general research support provided by Pfizer, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fury, M.G., Sherman, E., Ho, A. et al. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother Pharmacol 70, 121–128 (2012). https://doi.org/10.1007/s00280-012-1894-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1894-y