Abstract
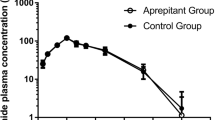
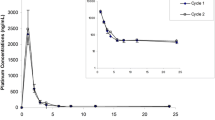
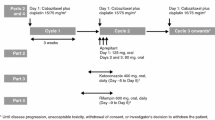
Background: Patients receiving the highly emetogenic high-dose chemotherapy regimen with cyclophosphamide, thiotepa and carboplatin (CTC) may benefit from the neurokin-1 receptor antagonist aprepitant in addition to standard anti-emetic therapy. As aprepitant has been shown to be a moderate inhibitor of the cytochrome P450 (CYP) 3A4 isoenzyme, its effect on the pharmacokinetics and metabolism of cyclophosphamide and thiotepa was evaluated. Moreover, preliminary results on the clinical efficacy of aprepitant in the CTC regimen are reported. Patients and methods: Six patients were enrolled in a protocol that employed a 4-day course of CTC high-dose chemotherapy with cyclophosphamide (1,500 mg/m2/day), thiotepa (120 mg/m2/day) and carboplatin (AUC 5 mg min/ml/day). Two patients received the tCTC protocol, which comprises two-third of the dose of CTC. In addition to standard anti-emetic therapy, the patients received aprepitant from one day before the start of their course until 3 days after chemotherapy. Blood samples were collected on days one and three of the course and analyzed for cyclophosphamide and its activated metabolite 4-hydroxycyclophosphamide, thiotepa and its main active metabolite tepa. The influence of aprepitant on the pharmacokinetics of cyclophosphamide and thiotepa was analyzed using a population pharmacokinetic analysis including a reference population of 49 patients receiving the same chemotherapy regimen without aprepitant and sampled under the same conditions. The frequency of nausea and vomiting in the six patients receiving CTC was compared with those of the last 22 consecutive patients receiving CTC chemotherapy without aprepitant. Inhibitory activity of aprepitant on cyclophosphamide and thiotepa metabolism was also tested in human liver microsomes. Results: In our patient population, the rate of autoinduction of cyclophosphamide (P=0.040) and the formation clearance of tepa (P<0.001) were reduced with 23% and 33% when aprepitant was co-administered, respectively. Exposures to the active metabolite 4-hydroxycyclophosphamide and tepa were therefore reduced (5% and 20%, respectively) in the presence of aprepitant. In human liver microsomes, the 50% inhibitory concentrations (IC50) of aprepitant for inhibition of cyclophosphamide (IC50=1.3 μg/ml) and thiotepa (IC50=0.27 μg/ml) metabolism were within the therapeutic range. Patients receiving aprepitant experienced less frequently CINV both during and after the CTC course compared with the reference population (nausea 3.7 days vs. 5.8 days, P=0.052; vomiting 0.5 days vs. 4.8 days, P<0.001). Conclusion: Aprepitant inhibited both cyclophosphamide and thiotepa metabolism, most probably due to inhibition of the CYP 3A4 and/or 2B6 isoenzymes. The effects of this interaction are, however, small compared to the total variability. Addition of aprepitant may provide superior protection against vomiting in patients receiving the highly emetogenic high-dose CTC chemotherapy.




Similar content being viewed by others
References
Beal SL, Sheiner LB (1998) User’s Guides, NONMEM Project Group. University of California at San Francisco, San Francisco
Chang TKH, Weber GF, Crespi CL, Waxman DJ (1993) Differential activation of cyclophosphamide and ifosfamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res 53:5629–5637
Craig AW, Jackson H (1995) The metabolism of 32P-labelled triethylenephosphoramide in relation to its anti-tumour activity. Br J Pharmacol 10:321–325
Dando TM, Perry CM (2004) Aprepitant. A review of its use in the prevention of chemotherapy-induced nausea and vomiting. Drugs 64:777–794
De Jonge ME, Huitema ADR, Rodenhuis S, Beijnen JH (2004) Integrated population pharmacokinetic model of both cyclophosphamide and thiotepa suggesting a mutual drug-drug interaction. J Pharmacokin Pharmacodyn 31:135–156
De Jonge ME, Van Dam SM, Hillebrand MJX, Rosing H, Huitema ADR, Rodenhuis S, Beijnen JH (2004) Simultaneous quantification of cyclophosphamide, 4-hydroxycyclophosphamide, N ,N′ ,N′″′ -triethylenethiophosphoramide (thiotepa) and N,N′ ,N″ -triethylenephosphoramide (tepa) in human plasma by high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC-MS/MS). J Mass Spectrom 39:233–254
De Wit R, Herrstedt J, Rapoport B, Carides AD, Carides G, Elmer M, Schmidt C, Evans JK, Horgan KJ (2003) Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy. J Clin Oncol 21:4105–4111
De Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, Schmidt C, Evans JK, Horgan KJ (2004) The oral NK1 antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomized, placebo-controlled phase II clinical trials. Eur J Cancer 40:403–410
Gervot L, Rochat B, Gautier JC, Bohnenstengel F, Kroemer H, de Berardinis V, Martin H, Beaune P, de Waziers I (1999) Human CYP2B6: expression, inducibility and catalytic activities. Pharmacogenetics 9:295–306
Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ, Singher LJ, Ettinger LJ, Gillespie A, Sam J et al (1989) Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res 49:736–741
Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, Chawla SP, Carides AD, Ianus J, Elmer ME, Evans JK, Beck K, Reines S, Horgan KJ Aprepitant Protocol 052 Study Group (2003) The oral neurokin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin. The aprepitant protocol 052 study group. J Clin Oncol 21:4112–4119
Huitema ADR, Kerbusch T, Tibben MM, Rodenhuis S, Beijnen JH (2000) Reduction of cyclophosphamide-bioactivation by thiotepa: critical sequence-dependency in high-dose chemotherapy regimens. Cancer Chemother Pharmacol 46:119–127
Huitema ADR, Spaander M, Mathôt RAA, Tibben MM, Holtkamp MJ, Beijnen JH, Rodenhuis S (2002) Relationship between exposure and toxicity in high-dose chemotherapy with cyclophosphamide, thiotepa and carboplatin. Ann Oncol 13:374–384
Jacobson PA, Green K, Birnbaum A, Remmel RP (2002) Cytochrome P450 isozymes 3A4 and 2B6 are involved in the in vitro human metabolism of thiotepa to tepa. Cancer Chemother Pharmacol 49:461–467
Lindley C, Hamilton G, McCune JS, Faucette S, Shord SS, Hawke RL, Wang H, Gilbert D, Jolley S, Yan B, LeCluyse EL (2002) The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos 30:814–822
Majumdar AK, McCrea JB, Panebianco DL, Hesney M, Dru J, Constanzer M, Goldberg MR, Murphy G, Gottesdiener KM, Lines CR, Petty KJ, Blum RA (2003) Effects of aprepitant on cytochrome P450 3A4 activity using midazolam as a probe. Clin Pharmacol Ther 74:150–156
Martin H, Sarsat JP, De Waziers I, Housset C, Balladur P, Beaune P, Albaladejo V, Lerche-Langrand C (2003) Induction of cytochrome P450 2B6 and 3A4 expression by phenobarbital and cyclophosphamide in cultured human liver slices. Pharm Res 20:557–568
McCrea JB, Majumar AK, Goldberg MR, Iwamoto M, Gargano C, Panebianco DL, Hesney M, Lines CR, Petty KJ, Deutsch PJ, Murphy MG, Gottesdiener KM, Goldwater DR, Blum RA (2003) Effects of the neurokin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther 74:17–24
Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, Julie Ma G, Eldridge K, Hipple A, Evans JK, Horgan KJ, Lawson F; Aprepitant Protocol 054 Study Group (2003) Addition of the neurokin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer 97:3090–3098
Product information: Emend(aprepitant) capsules. Merck&Co., Inc., March 2003
Ren S, Yang JS, Kalhorn TF, Slattery JT (1997) Oxidation of cyclophosphamide to 4-hydroxycyclophosphamide and deschloroethylcyclophosphamide in human liver microsomes. Cancer Res 57:4229–4235
Roy P, Yu LJ, Crespi CL, Waxman DJ (1999) Development of a substrate activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 profiles. Drug Metab Dispos 27:655–666
Rodenhuis S, Bontenbal M, Beex LV, Wagstaff J, Richel DJ, Nooij MA, Voest EE, Hupperets P, van Tinteren H, Peterse HL, TenVergert EM, de Vries EGE, Netherlands Working Party on Autologous Transplantation in Solid Tumors (2003) High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med 349:7–16
Rodenhuis S, de Wit R, de Mulder PH, Keizer HJ, Sleijfer DT, Lalisang RI, Bakker PJ, Mandjes I, Kooi M, de Vries EGE (1999) A multi-center prospective phase II study of high-dose chemotherapy in germ-cell cancer patients relapsing from complete remission. Ann Oncol 10:1467–1473
Rodenhuis S, Westermann A, Holtkamp MJ, Nooijen WJ, Baars JW, Van der Wall E, Slaper-Cortenbach IC, Schornagel JH (1996) Feasibility of multiple courses of high-dose cyclophosphamide, thiotepa, and carboplatin for breast cancer or germ cell cancer. J Clin Oncol 14:1473–1483
Schrama JG, Holtkamp MJ, Baars JW, Schornagel JH, Rodenhuis S (2003) Toxicity of the high-dose chemotherapy CTC regimen (cyclophosphamide, thiotepa, carboplatin): the Netherlands Cancer Institute experience. Br J Cancer 88:1831–1838
Shadle CR, Lee Y, Majumdar AK, Petty KJ, Gargano C, Bradstreet TE, Evans JK, Blum RA (2004) Evaluation of potential inductive effects of aprepitant on cytochrome P450 3A4 and 2C9 activity. J Clin Pharmacol 44:215–223
Teicher BA, Waxman DJ, Holden SA, Wang YY, Clarke L, Alvarez Sotomayor E, Jones SM, Frei E III (1989) Evidence for enzymatic activation and oxygen involvement in cytotoxicity and anti-tumor activity of N,N′ ,N′′ -Triethylenethiophosphoramide. Cancer Res 49:4996–5001
Van Maanen MJ, Huitema ADR, Beijnen JH (2000) Influence of co-medicated drugs on the biotransformation of thiotepa to tepa and thiotepa-mercapturate. Anticancer Res 20:1711–1716
Van Warmerdam LJC, Van Tellingen O, Maes RAA, Beijnen JH (1995) Validated method for the determination of carboplatin in biological fluids by Zeeman atomic absorption spectrometry. Fresenius J Anal Chem 352:777–781
Warr DG, Eisenberg P, Hesketh PJ, Gralla RJ, Raftopolous H, Gabriel M, Rodgers A, Klinger G, Hustad CM, Skobieranda F (2004) Effect of aprepitant for the prevention of nausea and vomiting after one cycle of moderately emetigenic chemotherapy: A randomized double-blind trial in 866 patients. Proc Am Soc Clin Oncol 23:abstract 8007
Yu L, Waxman J (1996) Role of cytochrome P450 in oxazaphosphorine metabolism. Deactivation via N-dechloroethylation and activation via 4-hydroxylation catalyzed by distinct subsets of rat liver cytochromes P450. Drug Metab Dispos 24:1254–1262
Acknowledgements
This research was supported with a grant from the Dutch Cancer Society (project NKI 2001–2420).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Jonge, M.E., Huitema, A.D.R., Holtkamp, M.J. et al. Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolism. Cancer Chemother Pharmacol 56, 370–378 (2005). https://doi.org/10.1007/s00280-005-1005-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-1005-4