Abstract
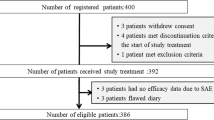
First-generation 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists (RAs) are currently the standard of care for prophylaxis against allo-HSCT-induced emesis. However, the efficacy of this combination in allo-HSCT recipients is not entirely satisfying. We sought to compare the efficacy of first-generation 5-HT3 RAs with that of second-generation 5-HT3 RAs in emesis prevention in allo-HSCT recipients. A total of 51 consecutive patients undergoing allo-HSCT for various hematological diseases in our institution were retrospectively reviewed. Patients who received daily first-generation 5-HT3 RAs, and 60-h palonosetron for emesis prophylaxis were stratified into the standard (n = 23) and palonosetron (n = 28) groups, respectively. Emesis severity and rescue therapy requirements in patients between these two groups were compared. Our results showed patients in standard and palonosetron groups had comparable severity of both acute and delayed emesis. However, 52.2 % of the patients in the standard group required rescue therapy, compared to only 21.4 % of the patients in the palonosetron group (p = 0.046). Subgroup analysis showed rescue therapy for acute emesis was required by 26.1 % of the patients in the standard group and by only 3.6 % of the patients in the palonosetron group (p = 0.037). In conclusion, palonosetron and first-generation 5-HT3 RAs were at least equally effective in emesis prophylaxis for allo-HSCT recipients. Patients receiving palonosetron, especially for acute emesis, required rescue therapy less frequently than those receiving first-generation 5-HT3 RAs.
Similar content being viewed by others
References
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G, Racil Z, Rowe JM, Russell N, Mohty M, Lowenberg B, Socie G, Niederwieser D, Ossenkoppele GJ (2012) The European LeukemiaNet AML working party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 9(10):579–590. doi:10.1038/nrclinonc.2012.150
Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, Aapro MS, Gandara D, Lindley CM (1997) Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol 15(1):103–109
Osoba D, Zee B, Warr D, Latreille J, Kaizer L, Pater J (1997) Effect of postchemotherapy nausea and vomiting on health-related quality of life. The quality of life and symptom control committees of the National Cancer Institute of Canada clinical trials group. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 5(4):307–313
Barrett A (1982) Total body irradiation (TBI) before bone marrow transplantation in leukaemia: a co-operative study from the European group for bone marrow transplantation. Br J Radiol 55(656):562–567
Al-Absi AI, Cooke CR, Wall BM, Sylvestre P, Ismail MK, Mya M (2010) Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc 42(9):3591–3593. doi:10.1016/j.transproceed.2010.08.066
van der Meij BS, de Graaf P, Wierdsma NJ, Langius JA, Janssen JJ, van Leeuwen PA, Visser OJ (2012) Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant. doi:10.1038/bmt.2012.124
Hesketh PJ (2008) Chemotherapy-induced nausea and vomiting. New Engl J Med 358(23):2482–2494. doi:10.1056/NEJMra0706547
Latreille J, Stewart D, Laberge F, Hoskins P, Rusthoven J, McMurtrie E, Warr D, Yelle L, Walde D, Shepherd F et al (1995) Dexamethasone improves the efficacy of granisetron in the first 24 h following high-dose cisplatin chemotherapy. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 3(5):307–312
Abbott B, Ippoliti C, Hecth D, Bruton J, Whaley B, Champlin R (2000) Granisetron (Kytril) plus dexamethasone for antiemetic control in bone marrow transplant patients receiving highly emetogenic chemotherapy with or without total body irradiation. Bone Marrow Transplant 25(12):1279–1283. doi:10.1038/sj.bmt.1702424
Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, Jakeman L, Parnes H, Whiting RL, Eglen RM (1995) The interaction of RS 25259-197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114(4):851–859
Rojas C, Thomas AG, Alt J, Stathis M, Zhang J, Rubenstein EB, Sebastiani S, Cantoreggi S, Slusher BS (2010) Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol 626(2–3):193–199. doi:10.1016/j.ejphar.2009.10.002
Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol Off J Eur Soc Med Oncol ESMO 17(9):1441–1449. doi:10.1093/annonc/mdl137
Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, Sakai H, Inoue K, Kitagawa C, Ogura T, Mitsuhashi S (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: A double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10(2):115–124. doi:10.1016/S1470-2045(08)70313-9
Di Renzo N, Montanini A, Mannina D, Dondi A, Muci S, Mancuso S, De Paolis MR, Plati C, Stelitano C, Patti C, Olivieri A, Liardo E, Buda G, Cantaffa R, Federico M (2011) Single-dose palonosetron for prevention of chemotherapy-induced nausea and vomiting in patients with aggressive non-Hodgkin’s lymphoma receiving moderately emetogenic chemotherapy containing steroids: results of a phase II study from the Gruppo Italiano per lo Studio dei Linfomi (GISL). Support Care Cancer Off J Multinatl Assoc Support Care Cancer 19(10):1505–1510. doi:10.1007/s00520-010-0974-y
Mattiuzzi GN, Cortes JE, Blamble DA, Bekele BN, Xiao L, Cabanillas M, Borthakur G, O’Brien S, Kantarjian H (2010) Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia. Cancer 11(24):5659–5666. doi:10.1002/cncr.25365
Giralt SA, Mangan KF, Maziarz RT, Bubalo JS, Beveridge R, Hurd DD, Mendoza FL, Rubenstein EB, DeGroot TJ, Schuster MW (2011) Three palonosetron regimens to prevent CINV in myeloma patients receiving multiple-day high-dose melphalan and hematopoietic stem cell transplantation. Ann Oncol Off J Eur Soc Med Oncol ESMO 22(4):939–946. doi:10.1093/annonc/mdq457
Musso M, Scalone R, Crescimanno A, Bonanno V, Polizzi V, Porretto F, Bianchini C, Perrone T (2010) Palonosetron and dexamethasone for prevention of nausea and vomiting in patients receiving high-dose chemotherapy with auto-SCT. Bone Marrow Transplant 45(1):123–127. doi:10.1038/bmt.2009.114
Ripaldi M, Parasole R, De Simone G, D’Amico MR, Migliorati R, Zanotta G, Loffredo G, Petruzziello F, Poggi V (2010) Palonosetron to prevent nausea and vomiting in children undergoing BMT: efficacy and safety. Bone Marrow Transplant 45(11):1663–1664. doi:10.1038/bmt.2010.23
Lopez-Jimenez J, Martin-Ballesteros E, Sureda A, Uralburu C, Lorenzo I, del Campo R, Fernandez C, Calbacho M, Garcia-Belmonte D, Fernandez G (2006) Chemotherapy-induced nausea and vomiting in acute leukemia and stem cell transplant patients: results of a multicenter, observational study. Haematologica 91(1):84–91
Rzepecki P, Pielichowski W, Oborska S, Barzal J, Mlot B (2009) Palonosetron in prevention of nausea and vomiting after highly emetogenic chemotherapy before haematopoietic stem cell transplantation-single center experience. Transplant Proc 41(8):3247–3249. doi:10.1016/j.transproceed.2009.07.071
Albany C, Brames MJ, Fausel C, Johnson CS, Picus J, Einhorn LH (2012) Randomized, double-blind, placebo-controlled, phase III cross-over study evaluating the oral neurokinin-1 antagonist aprepitant in combination with a 5HT3 receptor antagonist and dexamethasone in patients with germ cell tumors receiving 5-day cisplatin combination chemotherapy regimens: a hoosier oncology group study. J Clin Oncol Off J Am Soc Clin Oncol 30(32):3998–4003. doi:10.1200/JCO.2011.39.5558
Endo J, Iihara H, Yamada M, Yanase K, Kamiya F, Ito F, Funaguchi N, Ohno Y, Minatoguchi S, Itoh Y (2012) A randomized controlled non-inferiority study comparing the antiemetic effect between intravenous granisetron and oral azasetron based on estimated 5-HT3 receptor occupancy. Anticancer Res 32(9):3939–3947
Li P, Ma P, Wang Y, Tong W, Wang J, Wu C, Liu L (2012) Liquid chromatography–electrospray quadrupole linear ion trap mass spectrometry method for the quantitation of palonosetron in human plasma and urine: application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci 895–896:10–16. doi:10.1016/j.jchromb.2012.03.001
Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A (2004) Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol Off J Eur Soc Med Oncol ESMO 15(2):330–337
Boccia R, Grunberg S, Franco-Gonzales E, Rubenstein E, Voisin D (2013) Efficacy of oral palonosetron compared to intravenous palonosetron for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: a phase 3 trial. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 21(5):1453–1460. doi:10.1007/s00520-012-1691-5
Parfitt JR, Jayakumar S, Driman DK (2008) Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol 32(9):1367–1372
Conflict of interest
Chieh-Lin Jerry Teng received speaker fee from presenting data regarding palonosetron. Other authors declare that there is no conflict of interest.
Statement of informed consent
Informed consent from patients was not required for this study according to the regulation of Taichung Veterans General Hospital institutional review board.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chou, CW., Chen, YK., Yu, YB. et al. Palonosetron versus first-generation 5-hydroxytryptamine type 3 receptor antagonists for emesis prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation. Ann Hematol 93, 1225–1232 (2014). https://doi.org/10.1007/s00277-014-2038-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2038-8