Abstract
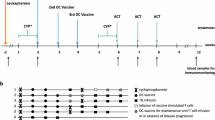
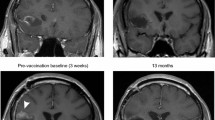
Antigen-specific immunotherapy was studied in a multi-institutional phase 1/2 study by combining decitabine (DAC) followed by an autologous dendritic cell (DC)/MAGE-A1, MAGE-A3 and NY-ESO-1 peptide vaccine in children with relapsed/refractory solid tumors. Patients aged 2.5–15 years with relapsed neuroblastoma, Ewing’s sarcoma, osteosarcoma and rhabdomyosarcoma were eligible to receive DAC followed by DC pulsed with overlapping peptides derived from full-length MAGE-A1, MAGE-A3 and NY-ESO-1. The primary endpoints were to assess the feasibility and tolerability of this regimen. Each of four cycles consisted of week 1: DAC 10 mg/m2/day for 5 days and weeks 2 and 3: DC vaccine once weekly. Fifteen patients were enrolled in the study, of which 10 were evaluable. Generation of DC was highly feasible for all enrolled patients. The treatment regimen was generally well tolerated, with the major toxicity being DAC-related myelosuppression in 5/10 patients. Six of nine patients developed a response to MAGE-A1, MAGE-A3 or NY-ESO-1 peptides post-vaccine. Due to limitations in number of cells available for analysis, controls infected with a virus encoding relevant genes have not been performed. Objective responses were documented in 1/10 patients who had a complete response. Of the two patients who had no evidence of disease at the time of treatment, one remains disease-free 2 years post-therapy, while the other experienced a relapse 10 months post-therapy. The chemoimmunotherapy approach using DAC/DC-CT vaccine is feasible, well tolerated and results in antitumor activity in some patients. Future trials to maximize the likelihood of T cell responses post-vaccine are warranted.


Similar content being viewed by others
Abbreviations
- ALT:
-
Alanine aminotransferase
- ANC:
-
Absolute neutrophil count
- AST:
-
Aspartate aminotransferase
- CGGs:
-
Cancer germline genes
- CTCAE:
-
Common terminology criteria for adverse events
- CTL:
-
Cytotoxic T lymphocytes
- C1W1:
-
Cycle1-week1
- DAC:
-
Decitabine
- DC:
-
Dendritic cell
- ECG:
-
Echocardiogram
- ES:
-
Ewing’s sarcoma
- GCSF:
-
Granulocyte colony-stimulating factor
- GFR:
-
Glomerular filtration rate
- GM-CSF:
-
Granulocyte macrophage colony-stimulating factor
- HLA:
-
Human leukocyte antigen
- NB:
-
Neuroblastoma
- OS:
-
Osteogenic sarcoma
- PBMCs:
-
Peripheral blood mononuclear cells
- RMS:
-
Rhabdomyosarcoma
References
Igne FH, Krammer PH (2002) Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol 71(6):907–920
Scanlan MJ, Simpson AJ, Old LJ (2004) The cancer/testis genes: review, standardization, and commentary. Cancer Immun 4:1
Pollack SM, Loggers ET, Rodler ET, Yee C, Jones RL (2011) Immune-based therapies for sarcoma. Sarcoma 2011:438940
Lee SY, Obata Y, Yoshida M, Stockert E, Williamson B, Jungbluth AA et al (2003) Immunomic analysis of human sarcoma. Proc Natl Acad Sci USA 100(5):2651–2656
Nicholaou T, Ebert L, Davis ID, Robson N, Klein O, Maraskovsky E et al (2006) Directions in the immune targeting of cancer: lessons learned from the cancer-testis Ag NY-ESO-1. Immunol Cell Biol 84(3):303–317
Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R et al (2005) Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother 54(4):400–406
Jacobs JF, Brasseur F, Hulsbergen-van de Kaa CA, van de Rakt MW, Figdor CG, Adema GJ et al (2007) Cancer-germline gene expression in pediatric solid tumors using quantitative real-time PCR. Int J Cancer 120(1):67–74
Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I (2000) XAGE-1, a new gene that is frequently expressed in Ewing’s sarcoma. Cancer Res 60(17):4752–4755
Foell JL, Hesse M, Volkmer I, Schmiedel BJ, Neumann I, Staege MS (2008) Membrane-associated phospholipase A1 beta (LIPI) Is an Ewing tumour-associated cancer/testis antigen. Pediatr Blood Cancer 51(2):228–234
Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N et al (1994) Expression of the MAGE-1 tumor antigen is up-regulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res 54(7):1766–1771
Adair SJ, Hogan KT (2009) Treatment of ovarian cancer cell lines with 5-aza-2′-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol Immunother 58(4):589–601
George RE, Lahti JM, Adamson PC, Zhu K, Finkelstein D, Ingle AM et al (2010) Phase I study of decitabine with doxorubicin and cyclophosphamide in children with neuroblastoma and other solid tumors: a Children’s Oncology Group study. Pediatr Blood Cancer 55(4):629–638
Yang H, Hoshino K, Sanchez-Gonzalez B, Kantarjian H, Garcia-Manero G (2005) Antileukemia activity of the combination of 5-aza-2′-deoxycytidine with valproic acid. Leuk Res 29(7):739–748
Issa JP, Gharibyan V, Cortes J, Jelinek J, Morris G, Verstovsek S et al (2005) Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol 23(17):3948–3956
Bao L, Dunham K, Lucas K (2011) MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother 60(9):1299–1307
Krishnadas DK, Bao L, Bai F, Chencheri SC, Lucas K (2014) Decitabine facilitates immune recognition of sarcoma cells by upregulating CT antigens, MHC molecules, and ICAM-1. Tumour Biol 35(6):5753–5762
Chianese-Bullock KA, Pressley J, Garbee C, Hibbitts S, Murphy C, Yamshchikov G et al (2005) MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol 174(5):3080–3086
Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q et al (2004) Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA 101(29):10697–10702
Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A et al (2014) Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res 2(1):37–49
Schwartz GJ, Gauthier B (1985) A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106(3):522–526
Krishnadas DK, Shapiro T, Lucas K (2013) Complete remission following decitabine/dendritic cell vaccine for relapsed neuroblastoma. Pediatrics 131(1):e336–e341
Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C (2000) Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95(8):2484–2490
Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G et al (2010) Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 116(11):1908–1918
Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M et al (2007) Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110(1):201–210
Zhu Y, Chen L (2014) CD137 as a biomarker for tumor-reactive T cells: finding gold in the desert. Clin Cancer Res 20(1):3–5
Akers SN, Odunsi K, Karpf AR (2010) Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy. Future Oncol 6(5):717–732
Mackensen A, Herbst B, Chen JL, Köhler G, Noppen C, Herr W, Spagnoli GC et al (2000) Phase I study in melanoma patients of a vaccine with peptide-pulsed dendritic cells generated in vitro from CD34(+) hematopoietic progenitor cells. Int J Cancer 86(3):385–392
Bender A, Karbach J, Neumann A, Jäger D, Al-Batran SE, Atmaca A, Weidmann E et al (2007) LUD 00-009: phase 1 study of intensive course immunization with NY-ESO-1 peptides in HLA-A2 positive patients with NY-ESO-1-expressing cancer. Cancer Immun 7:16
Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J et al (2009) Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor–secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol 27(35):5911–5918
King IL, Kroenke MA, Segal BM (2010) GM-CSF-dependent, CD103+ dermal dendritic cells play a critical role in Th effector cell differentiation after subcutaneous immunization. J Exp Med 207(5):953–961
Vadhan-Raj S, Broxmeyer HE, Hittelman WN, Papadopoulos NE, Chawla SP, Fenoglio C et al (1992) Abrogating chemotherapy-induced myelosuppression by recombinant granulocyte-macrophage colony-stimulating factor in patients with sarcoma: protection at the progenitor cell level. J Clin Oncol 10(8):1266–1277
Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L et al (2011) Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood 117(3):788–797
Rapoport AP, Stadtmauer EA, Aqui N, Vogl D, Chew A, Fang HB et al (2009) Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res 15(13):4499–4507
Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K et al (2012) Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 18(23):6497–6508
Acknowledgments
We thank the clinical research staff at the Dana-Farber Cancer Institute (DFCI), Kosair Charities Pediatric Clinical Research Unit and Penn State Children’s Hospital. The work was supported by funds from Hyundai Hope on Wheels, Solving Kids’ Cancer, The Andrew McDonough B + Foundation, the Pierce Phillips Charity and the Dana-Farber Cancer Institute (DFCI) Neuroblastoma Fund. We thank all the patients enrolled in this trial and their families for their participation in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Krishnadas, D.K., Shusterman, S., Bai, F. et al. A phase I trial combining decitabine/dendritic cell vaccine targeting MAGE-A1, MAGE-A3 and NY-ESO-1 for children with relapsed or therapy-refractory neuroblastoma and sarcoma. Cancer Immunol Immunother 64, 1251–1260 (2015). https://doi.org/10.1007/s00262-015-1731-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1731-3